Abstract
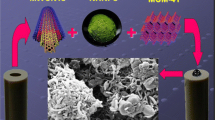
In this study, a novel method was developed to fabricate a glassy carbon electrode (GCE) modified by a composition of multi-walled carbon nanotubes (MWCNTs) and Fe3O4@MCM-48-SO3H nanoparticles (MWCNTs-Fe3O4@MCM-48-SO3H/GCE) as a sensitive anionic composite layer for simultaneous determination of norepinephrine (NE) and tyrosine (Tyr) in the presence of ascorbic acid (AA). Electrochemical behavior of the electrode was studied using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) and chronoamperometry (CA) techniques. Under the optimum conditions, the modified electrode provides a linear anodic peak versus NE concentrations in the range of 0.4–600 μM with a detection limit of 0.19 μM and Tyr concentrations in the range of 0.9–400 μM with a detection limit of 0.28 μM, respectively, using the differential pulse voltammetry method. The modified electrode has been successfully applied for the determination of NE and Tyr in real samples.






Similar content being viewed by others
References
Fauci AS, Kasper DL, Longo DL, Braunwald E, Hauser L, Jameson JL, Loscalzo J (2008) Harrison’s principles of internal medicine, vol 2, 17th edn. Wiley, New York, pp 2470–2471. https://doi.org/10.1111/j.1445-5994.2008.01837.x
Babaei A, Zendehdel M, Khalilzadeh B, Abnosi B (2010) A new sensor for simultaneous determination of tyrosine and dopamine using iron(III) doped zeolite modified carbon paste electrode. Chin J Chem 28:1967–1972. https://doi.org/10.1002/cjoc.201090328
Azuma Y, Maekawa M, Kuwabara Y, Nakajima T, Taniguchi K, Kanno T (1989) Determination of branched-chain amino acids and tyrosine in serum of patients with various hepatic diseases, and its clinical usefulness. Clin Chem 3:1399
Sabine L, Jean PG, Joelle S, Bernard B (1997) Determination of the l-DOPA/l-tyrosine ratio in human plasma by high-performance liquid chromatography: usefulness as a marker in metastatic malignant melanoma. J Chromatography B 696:9
Andrensek S, Golc-Wondra A, Prosek M (2003) Determination of phenylalanine and tyrosine by liquid chromatography/mass spectrometry. J AOAC Int 86:753–758
Deng C, Deng Y, Wang B, Yang X (2002) Gas chromatography-mass spectrometry method for determination of phenylalanine and tyrosine in neonatal blood spots. J Chromatogr B Analyt Technol Biomed Life Sci 780:407–413
Grenier A, Laberge C (1974) A modified automated fluorometric method for tyrosine determination in blood spotted on paper: a mass screening procedure for tyrosinemia. Clin Chim Acta 57:71–75. https://doi.org/10.1016/0009-8981(74)90179-X
Jin G-P, Lin X-Q (2004) The electrochemical behavior and amperometric determination of tyrosine and tryptophan at a glassy carbon electrode modified with butyrylcholine. Electrochem Commun 6:454–460. https://doi.org/10.1016/j.elecom.2004.03.006
D’Souza OJ, Mascarenhas RJ, Satpati AK, Aiman LV, Mekhalif Z (2015) Electrocatalytic oxidation of L-tyrosine at carboxylic acid functionalized multi-walled carbon nanotubes modified carbon paste electrode. Ionics 22:404–414. https://doi.org/10.1007/s11581-015-1552-6
Katzung BG (2004) Basic & clinical pharmacology, 9th edn. McGraw Hill, New York, pp 132–133
Zhu M, Huang X, Li J, Shen H (1997) Peroxidase-based spectrophotometric methods for the determination of ascorbic acid, norepinephrine, epinephrine, dopamine and levodopa. Anal Chim Acta 357:261–267. https://doi.org/10.1016/S0003-2670(97)00561-8
Warnhoff M (1984) Simultaneous determination of norepinephrine, dopamine, 5-hydroxytryptamine and their main metabolites in rat brain using high-performance liquid chromatography with electrochemical detection: enzymatic hydrolysis of metabolites prior to chromatography. J Chromatogr 307:271–281. https://doi.org/10.1016/S0378-4347(00)84099-2
Wei S, Song G, Lin J-M (2005) Separation and determination of norepinephrine, epinephrine and isoprinaline enantiomers by capillary electrophoresis in pharmaceutical formulation and human serum. J Chromatogr A 1098:166–171. https://doi.org/10.1016/j.chroma.2005.08.038
Renzini V, Brunori CA, Valori C (1970) A sensitive and specific fluorimetric method for the determination of noradrenalin and adrenalin in human plasma. Clin Chim Acta 30:587–594. https://doi.org/10.1016/0009-8981(70)90249-4
Mazloum-Ardakani M, Sheikh-Mohseni MA, Mirjalili B-F (2014) Nanomolar detection limit for determination of norepinephrine in the presence of acetaminophen and tryptophan using carbon nanotube-based electrochemical sensor. Ionics 20:431–437. https://doi.org/10.1007/s11581-013-0984-0
Beitollahi H, Mohadesi A, Khalilizadeh-Mahani S (2012) New voltammetric strategy for simultaneous determination of norepinephrine, acetaminophen, and folic acid using a 5-amino-3′,4′-dimethoxy-biphenyl-2-ol/carbon nanotube paste electrode. Ionics 18:703–710. https://doi.org/10.1007/s11581-012-0669-0
Murai S, Saito H, Masuda Y, Itolh T (1988) Rapid determination of norepinephrine, dopamine, serotonin, their precursor amino acids, and related metabolites in discrete brain areas of mice within ten minutes by HPLC with electrochemical detection. J Neurochem 50:473–479. https://doi.org/10.1111/j.1471-4159.1988.tb02935.x
Jeong H, Jeon S (2008) Determination of dopamine in the presence of ascorbic acid by nafion and single-walled carbon nanotube film modified on carbon fiber microelectrode. Sensors 8:6924–6935. https://doi.org/10.3390/s8116924
Rodríguez MC, Rubianes MD, Rivas GA (2008) Highly selective determination of dopamine in the presence of ascorbic acid and serotonin at glassy carbon electrodes modified with carbon nanotubes dispersed in polyethylenimine. J Nanosci Nanotechnol 8:6003–6009. https://doi.org/10.1166/jnn.2008.466
Vidya H, Kumara Swamy BE (2015) Voltammetric determination of dopamine in the presence of ascorbic acid and uric acid at sodium dodecyl sulphate/reduced graphene oxide modified carbon paste electrode. J Mol Liq 211:705–711. https://doi.org/10.1016/j.molliq.2015.07.011
Hu C, Hu S (2009) Carbon nanotube-based electrochemical sensors: principles and applications in biomedical systems. J Sens 2009:187615, 40 pages. https://doi.org/10.1155/2009/187615
Afrasiabi M, Kianipour S, Babaei A, Nasimi AA, Shabanian M (2013) A new sensor based on glassy carbon electrode modified with nanocomposite for simultaneous determination of acetaminophen, ascorbic acid and uric acid. J Saudi Chem Soc 20:S480–S487. https://doi.org/10.1016/j.jscs.2013.02.002
Baghayeri M, Sedrpoushan A, Mohammadi A, Heidari M (2017) A non-enzymatic glucose sensor based on NiO nanoparticles/functionalized SBA 15/MWCNT-modified carbon paste electrode. Ionics 23:1553–1562. https://doi.org/10.1007/s11581-016-1964-y
Babaei A, Yousefi A, Afrasiabi M, Shabanian M (2015) A sensitive simultaneous determination of dopamine, acetaminophen and indomethacin on a glassy carbon electrode coated with a new composite of MCM-41 molecular sieve/nickel hydroxide nanoparticles/multiwalled carbon nanotubes. J Electroanal Chem 740:28–36. https://doi.org/10.1016/j.jelechem.2014.12.042
Ajayan PM, Zhou OZ (2001) Applications of carbon nanotubes. Top Appl Phys 80:391–425
Vairavapandian D, Vichchulada P, Lay MD (2008) Preparation and modification of carbon nanotubes: review of recent advances and applications in catalysis and sensing. Anal Chim Acta 626:119–129. https://doi.org/10.1016/j.aca.2008.07.052
Gong K, Du F, Xia Z, Durstock M, Dai L (2009) Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323:760–764
Satyanarayana M, Yugender Goud K, Koteshwara Reddy K, Vengatajalabathy Gobi K (2017) Conducting polymer-layered carbon nanotube as sensor Interface for electrochemical detection of dacarbazine in-vitro. Electrocatalysis 8:214–223. https://doi.org/10.1007/s12678-017-0357-y
Babaei A, Afrasiabi M (2015) A glassy carbon electrode modified with MCM-41/nickel hydroxide nanoparticle/multiwalled carbon nanotube composite as a sensor for the simultaneous determination of dopamine, piroxicam, and cefixime. Ionics 21:1731–1740. https://doi.org/10.1007/s11581-014-1339-1
Anderson MW (1997) Simplified description of MCM-48. Zeolites 19:220–227
Vallet-Regi M, Balas F (2008) Silica materials for medical applications. Open Biomed Eng J 2:1–9. https://doi.org/10.2174/1874120700802010001
Matei D, Cursaru DL, Mihai S (2016) Preparation of MCM-48 mesoporous molecular sieve influence of preparation conditions on the structural properties. Dig J Nanomater Biostruct 11:271–276
Kefayati H, Golshekan M, Shariati S, Bagheri M (2015) Fe3O4@MCM-48–SO3H: an efficient magnetically separable nanocatalyst for the synthesis of benzo[f]chromeno[2,3-d]pyrimidinones. Chin J Catal 36:572–578. https://doi.org/10.1016/S1872-2067(14)60286-2
Bard AJ, Faulkner LR (2001) Electrochemical methods. Fundamentals and applications, 2nd edn. Wiley, New York, p 229
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28. https://doi.org/10.1016/S0022-0728(79)80075-3
Wang X, Cheng Y, You Z, Sha H, Gong S, Liu J, Sun W (2015) Sensitive electrochemical determination of oxalic acid in spinach samples by a graphene-modified carbon ionic liquid electrode. Ionics 21:877–884. https://doi.org/10.1007/s11581-014-1233-x
Afrasiabi M, Kianipour S (2015) Simultaneous determination of ascorbic acid, uric acid and acetaminophen on a glassy carbon electrode coated with a novel single walled carbon nanotubes/chitosan/MCM-41 composite. Anal Bioanal Electrochem 7:331–343
Molaakbari E, Mostafavi A, Beitollahi H (2014) First electrochemical report for simultaneous determination of norepinephrine, tyrosine and nicotine using a nanostructure based sensor. Electroanalysis 26:2252–2260. https://doi.org/10.1002/elan.201400338
Taei M, Ramazani G (2014) Simultaneous determination of norepinephrine, acetaminophen and tyrosine by differential pulse voltammetry using Au-nanoparticles/poly(2-amino-2-hydroxymethyl-propane-1,3-diol)film modified glassy carbon electrode. Colloids Surf B: Biointerfaces 123:23–32. https://doi.org/10.1016/j.colsurfb.2014.09.005
Behpour M, Masoum S, Meshki M (2013) Study and electrochemical determination of tyrosine at graphene nanosheets composite film modified glassy carbon electrode. J Nanostruct 3:243–251
Quintana C, Suárez S, Hernández L (2010) Nanostructures on gold electrodes for the development of an l-tyrosine electrochemical sensor based on host–guest supramolecular interactions. Sensors Actuators B 149:129–135. https://doi.org/10.1016/j.snb.2010.06.011
Fan Y, Liu JH, Lu HT, Zhang Q (2011) Electrochemistry and voltammetric determination of L-tryptophan and L-tyrosine using a glassy carbon electrode modified with a Nafion/TiO2-graphene composite film. Microchim Acta 173:241–247. https://doi.org/10.1007/s00604-011-0556-9
Arvand M, Gholizadeh TM (2013) Simultaneous voltammetric determination of tyrosine and paracetamol using a carbon nanotube-graphene nanosheet nanocomposite modified electrode in human blood serum and pharmaceuticals. Colloids Surf B: Biointerfaces 103:84–93. https://doi.org/10.1016/j.colsurfb.2012.10.024
Mazloum-Ardakani M, Beitollahi M, Sheikh-Mohseni MA, Naeimi H, Taghaviniac N (2010) Novel nanostructure electrochemical sensor for electrocatalytic determination of norepinephrine in the presence of high concentrations of acetaminophen and folic acid. Appl Catal A Gen 378:195–201. https://doi.org/10.1016/j.apcata.2010.02.019
Ma M, Chen M, Li X, Purushothaman A, Li F (2012) Electrochemical detection of norepinephrine in the presence of epinephrine, uric acid and ascorbic acid using a graphene-modified electrode. Int J Electrochem Sci 7:991–1000
Wang J, Li M, Shi Z, Li N, Gu Z (2002) Electrocatalytic oxidation of norepinephrine at a glassy carbon electrode modified with single wall carbon nanotubes. Electroanalysis 14:225–230
Funding
The authors would like to gratefully acknowledge the research council of Arak University for providing financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 446 kb)
Rights and permissions
About this article
Cite this article
Yousefi, A., Babaei, A. A new sensor based on glassy carbon electrode modified with Fe3O4@MCM-48-SO3H/multi-wall carbon nanotubes composite for simultaneous determination of norepinephrine and tyrosine in the presence of ascorbic acid. Ionics 25, 2845–2856 (2019). https://doi.org/10.1007/s11581-018-2815-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2815-9