Abstract
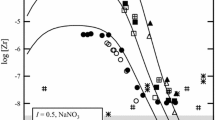
The solubility of Zr(OH)4(am)—in other words hydrated Zr(IV) oxyhydroxide—is determined by means of coulometric titration (CT), and colloids are detected by laser-induced breakdown when the solubility limit is exceeded. Our results at pH 3–8 demonstrate that the solubility of Zr(OH)4(am) is several orders of magnitude higher than reported classical solubility data for acidic solutions, determined from undersaturation with a less soluble microcrystalline Zr(IV) oxide precipitate. Analysis of extended X-ray absorption fine structure (EXAFS) data shows that the microcrystalline colloids in a 0.1 mol l−1 Zr aqueous solution at pH 0.2 contain tetrameric units, similar to those present in the structure of ZrOCl2.8H2O. Characterization of the CT solutions by means of EXAFS shows that oligomeric species form as the solubility limit is approached. The current lack of data on equilibrium constants for polynuclear hydroxide complexes prohibits the use of a realistic speciation model to describe the solubility of pH-dependent Zr(OH)4(am). However, the solubility curve is obtained using the mononuclear hydrolysis constants estimated in the present paper, along with the solubility constant (logK′sp=−49.9±0.5 in 0.5 mol l−1 NaCl; logK°sp=−53.1±0.5 at I=0).









Similar content being viewed by others
References
Hummel W, Berner U, Curti E, Pearson FJ, Thoenen T (2002) Radiochim Acta 90:805–813
Ekberg C, Kallvenius G, Albinsson Y, Brown PL (2004) J Solution Chem 33:47–79
Curti E, Degueldre C (2002) Radiochim Acta 90:801–804
Neck V, Müller R, Bouby M, Altmaier M, Rothe J, Denecke MA, Kim JI (2002) Radiochim Acta 90:485–494
Cho H-R, Walther C, Neck V, Fanghänel T (2004) unpublished results
Kovalenko PN, Bagdasarov KN (1961) Russ J Inorg Chem 6:272–275
Ciavatta L (1980) Ann Chim (Rome) 70:551
Grenthe I, Fuger J, Konings RJM, Lemire RJ, Muller AB, Nguyen-Trung C, Wanner H (1992) Chemical thermodynamics of uranium. Elsevier, Amsterdam
Neck V, Kim JI (2001) Radiochim Acta 89:1–16
Neck V, Kim JI (2000) Radiochim Acta 88:815–822
Shannon RD (1976) Acta Cryst 32:751
Neck V, Kim JI, Seidel BS, Marquardt CM, Dardenne K, Jensen MP, Hauser W (2001) Radiochim Acta 89:1–8
Neck V, Altmaier M, Müller R, Bauer A, Fanghänel T, Kim JI (2003) Radiochim Acta 91:253–262
Bitea C, Müller R, Neck V, Walther C, Kim JI (2003) Colloids Surf A 217:63–70
Scherbaum FJ, Knopp R, Kim JI (1996) Appl Phys B 63:299–306
Kitamori T, Yokose K, Sakagami M, Sawada T (1989) Jpn J Appl Phys 28:1195–1198
Walther C, Cho H-R, Fanghänel T (2004) Appl Phys Lett 85:6329–6331
Walther C, Bitea C, Hauser W, Kim JI, Scherbaum FJ (2002) Nucl Instrum Meth B 195:374–388
Denecke MA, Rothe J, Dardenne K, Blank H, Hormes J (2005) Phys Scr T115:1001
Sayers DE, Bunker BA (1988) In: Koningsberger DC, Prins R (eds) X-ray absorption: techniques of EXAFS, SEXAFS and XANES. Wiley, New York, pp 211–253
Ressler T (1997) J Phys IV 7-C2:269
Stern EA, Newville M, Ravel B, Yacoby Y, Haskel D (1995) Physica B 208/209:117–120
Gualtieri A, Norby P, Hanson J, Hriljac J (1996) J Appl Cryst 29:707–713
Ankudinov AL, Ravel B, Rehr JJ, Conradson SD (1998) Phys Rev B 58:7565–7576
Ankudinov AL, Rehr JJ (1997) Phys Rev B 56:1712
Smith DK, Newkirk HW (1965) Acta Cryst 18:983–991
Adam J, Rodgers MD (1959) Acta Cryst 12:951
Lee PA, Citrin PH, Eisenberger P, Kincaid BM (1981) Rev Mod Phys 53:769–806
Newville M (1995) FEFFIT—using FEFF to model XAFS data. Department of Physics, FM-15, University of Washington, Seattle, WA
Mak TCW (1967) Can J Chem 46:3491–3497
Denecke MA, Geckeis H, Pohlmann C, Rothe J, Degering D (2000) Radiochim Acta 88:639–643
Degueldre C, Pfeiffer H-R, Alexander W, Wernli B, Bruetsch R (1996) Appl Geochem 11:677–695
Li P, Chen I-W, Penner-Hahn JE (1993) Phys Rev B 48(14):10063–10073
Rothe J, Denecke MA, Neck V, Müller R, Kim JI (2002) Inorg Chem 41:249–258
Rothe J, Walther C, Denecke MA, Fanghänel Th (2004) Inorg Chem 43:4708–4718
Winterer M (2000) J Appl Phys 88:5635–5644
Singhal A, Toth LM, Lin JS, Affholter K (1996) J Am Chem Soc 118:11529–11534
Hagfeldt C, Kessler V, Persson I (2004) Dalton Trans 2142–2151
Muha GM, Vaughan PA (1960) J Chem Phys 33:194–199
Southton PD, Bartlett JR, Woolfrey JL, Ben-Nissan B (2002) Chem Mater 14:4313–4319
Zyuzin DA, Moroz EM, Ivanova AS, Shmakov AN, Kustova GN (2004) Kinet Catal 45(5):780–783
Ohtaka O, Yamanaka T, Kume S, Hara N, Asano H, Izumi F (1990) Proc Jpn Acad B 66:193
Teufer G (1962) Acta Cryst 15:1187
MacDermott TE (1973) Coord Chem Rev 11:1–20
Adair JH, Denkewicz RP, Arriagada FJ (1987) Ceram Trans 1:135–145
Bilinski H, Branica M, Sillen LG (1966) Acta Chem Scand 20:853–861
Bundschuh T, Knopp R, Müller R, Kim JI, Neck V, Fanghänel T (2000) Radiochim Acta 88:625–629
Schindler PW (1967) Adv Chem Ser 67:196
Ekberg C, Brown P, Comarmond J, Albinsson Y (2001) Mater Res Soc Symp 663:1091–1099
Tulock JJ, Blanchard GJ (2002) J Phys Chem B 106:3568–3575
Hu MZC, Zielke JT, Lin JS, Byers CH (1999) J Mater Res 14:103–113
Knopp R, Neck V, Kim JI (1999) Radiochim Acta 86:101–108
Pouchon MA, Curti E, Degueldre C, Tobler LU (2001) Prog Nucl Energy 38:443–446
Acknowledgements
We gratefully acknowledge the beamtime allotment by ANKA/ISS for measuring the monoclinic ZrO2 reference sample and experimental assistance by S. Mangold. We also acknowledge the analytic group of INE for ICP-MS concentration measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, HR., Walther, C., Rothe, J. et al. Combined LIBD and XAFS investigation of the formation and structure of Zr(IV) colloids. Anal Bioanal Chem 383, 28–40 (2005). https://doi.org/10.1007/s00216-005-3354-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-3354-6