Abstract
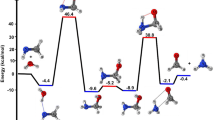
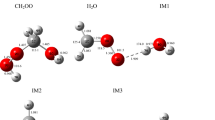
A comprehensive theoretical study on the role of water clusters ((H2O) n , n = 3–5) in the reaction of CH2OO with water vapour is done at the CCSD(T)//M06-2X/6-311 + G(2df,2p) level of theory. Our simulation results show that the contribution of entropic effect is significantly increased with the increase in the number of water molecules involved. The main products of CH2OO reaction with water vapour are α-hydroxymethyl hydroperoxide and its isomers. Contrary to the reaction of CH2OO with unimolecular water (10.91 kcal mol−1), the barriers of reactions with bi- and trimolecular water are further reduced to 5.25 and 4.75 kcal mol−1, respectively. Among these three reactions, the predominant pathway is the reaction of CH2OO with bimolecular water owing to its lower barrier and the high concentration of (H2O)2 ([(H2O)2] ≈ 2.47 × 1014 molecules cm−3). The presence of water clusters exhibits a dramatic catalytic effect in the CH2OO reaction with bimolecular water as it lowers the energy barrier by 4.0 kcal mol−1. The relative reaction rates, which are determined at temperatures from 273 to 323 K and 85 % relative humidity, reveal that the most favourable pathway for the atmospheric removal of CH2OO is indeed via its reaction with bimolecular water.









Similar content being viewed by others
References
Smith MC, Chang CH, Chao W, Lin LC, Takahashi K, Boering KA, Lin JJM (2015) J Phys Chem Lett 6:2708
Nguyen TN, Putikam R, Lin MC (2015) J Chem Phys 142:124312
Vereecken L, Harder H, Novelli A (2012) Phys Chem Chem Phys 14:14682
Criegee R, Wenner G (1949) Chem Ber 82:9
Su YT, Huang YH, Witek HA, Lee YP (2013) Science 340:174
Anglada JM, González J, Torrent-Sucarrat M (2011) Phys Chem Chem Phys 13:13034
Li J, Carter S, Bowman JM, Dawes R, Xie DQ, Guo H (2014) J Phys Chem Lett 5:2364
Li J, Guo H (2015) J Phys Chem A. doi:10.1021/acs.jpca.5b08491
Kuwata KT, Guinn EJ, Hermes MR, Fernandez JA, Mathison JM, Huang K (2015) J Phys Chem A 119:10316
Stone D, Blitz M, Daubney L, Howes NUM, Seakins P (2014) Phys Chem Chem Phys 16:1139
Berndt T, Kaethner R, Voigtländer J, Stratmann F, Pfeifle M, Reichle P, Sipilä M, Kulmala M, Olzmann M (2015) Phys Chem Chem Phys 17:19862
Berndt T, Voigtländer J, Stratmann F, Junninen H, Mauldin IIIRL, Sipilä M, Kulmala M, Herrmann H (2014) Phys Chem Chem Phys 16:19130
Long B, Tan XF, Long ZW, Wang YB, Ren DS, Zhang WJ (2011) J Phys Chem A 115:6559
Welz O, Savee JD, Osborn DL, Vasu SS, Percival CJ, Shallcross DE, Taatjes CA (2012) Science 335:204
Ouyang B, McLeod MW, Jones RL, Bloss WJ (2013) Phys Chem Chem Phys 15:17070
Zhang WC, Du BN, Qin ZL (2014) J Phys Chem A 118:4797
Zhang TL, Wang R, Chen H, Min ST, Wang ZY, Zhao CB, Xu Q, Jin LX, Wang WL, Wang ZQ (2015) Phys Chem Chem Phys 17:15046
Ryzhkov AB, Ariya PA (2004) Phys Chem Chem Phys 6:5042
Inaba S (2014) J Phys Chem A 118:3026
Ryzhkov AB, Ariya PA (2006) Chem Phys Lett 419:479
Chao W, Hsieh JT, Chang CH, Lin JJM (2015) Science 347:751
Lewis TR, Blitz MA, Heard DE, Seakins PW (2015) Phys Chem Chem Phys 17:4859
Frisch MJ et al (2009) Gaussian 09, revision C.01. Gaussian Inc, Wallingford, CT
Zhao Y, Truhlar DG (2006) J Chem Phys 125:194101
Zheng JJ, Truhlar DG (2009) J Phys Chem A 113:11919
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215
Miliordos E, Xantheas SS (2016) Angew Chem 128:1027
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Fukui K (1981) Acc Chem Res 14:363
Page M, Mclver JW (1988) J Chem Phys 88:922
Mendes J, Zhou CW, Curran HJ (2013) J Phys Chem A 117:4515
Mendes J, Zhou CW, Curran HJ (2013) J Phys Chem A 117:14006
Eckart C (1930) Phys Rev 35:1303
Johnston HS, Heicklen J (1962) J Phys Chem 66:532
Garrett BC, Truhlar DG (1979) J Phys Chem 83:2921
Duncan WT, Bell RL, Truong TN (1998) J Comput Chem 19:1039
Zhang TL, Wang WL, Zhang P, Lü J, Zhang Y (2011) Phys Chem Chem Phys 13:20794
McCarthy MC, Cheng L, Crabtree KN, Martinez O, Nguyen TL, Womack CC, Stanton JF (2013) J Phys Chem Lett 4:4133
Nakajima M, Endo Y (2013) J Chem Phys 139:101103
Chen L, Wang WL, Wang WN, Liu YL, Liu FY, Liu N, Wang BZ (2016) Theor Chem Acc 135:131
Crehuet R, Anglada JM, Bofill JM (2001) Chem Eur J 7:2227
Lin LC, Chang HT, Chang CH, Chao W, Smith MC, Chang CH, Lin JJM, Takahashi K (2016) Phys Chem Chem Phys 18:4557
Liu JJ, Fang S, Bing Q, Tao FM, Liu JY (2016) Comput Theor Chem 1076:11
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No: 21473108, 21473107), Shaanxi Innovative Team of Key Science and Technology (2013KCT-17) and the Fundamental Research Funds for the Central Universities (GK201601005).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, L., Wang, W., Zhou, L. et al. Role of water clusters in the reaction of the simplest Criegee intermediate CH2OO with water vapour. Theor Chem Acc 135, 252 (2016). https://doi.org/10.1007/s00214-016-1998-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1998-2