Abstract
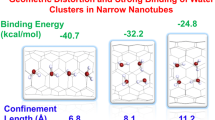
Water present in the nanoscale confined medium like the hydrophobic interior of the carbon nanotubes (CNT) is known to extol unique properties that depart largely from their behavior in the bulk form. Considering suitable model systems that structurally resemble single unit of the CNT, we demonstrate that two unique parameters namely the local nanoscale curvature and the confinement length are of cardinal importance in governing the structural and electronic properties of water molecule present inside CNT in a general manner. Water molecule encapsulated between the model systems that offer both the above two effects exhibits dramatic trend in the interaction energy with respect to the variation of these parameters. In relation to the curvature of the model system, we propose three different regimes where water molecule experiences a distinct trend in the stabilization energy. Similarly, a confinement distance of 6 Å also marks as a borderline for the distinct manifestations in the stabilization energy of the water molecule. These two parameters also play a key role in governing the significant variations in the structural parameters, Mulliken charges, and red and blue shift in the O–H vibrational frequencies of the encapsulated water molecule. There seems to be interplay between curvature and confinement in deciding the electronic properties of water in the nanoscale confinement.





Similar content being viewed by others
References
Schlichting I, Berendzen J, Chu K, Stock AM, Maves SA, Benson DE, Sweet BM, Ringe D, Petsko GA, Sligar SG (2000) Science 287:1615
Taraphder S, Hummer G (2003) J Am Chem Soc 125:3931
de Groot BL, Grubmüller H (2001) Science 294:2353
Hub JS, de Groot BL (2008) Proc Natl Acad Sci USA 105:1198
Jensen MO, Dror RO, Xu H, Borhani DW, Arkin IT, Eastwood MP, Shaw DE (2008) Proc Natl Acad Sci USA 105:14430
Hinds BJ, Chopra N, Rantell T, Andrews R, Gavalas V, Bacha LG (2004) Science 303:62
Holt JK, Park HG, Wang Y, Stadermann M, Artyukhin AB, Grigoropoulos CP, Noy A, Bakajin O (2006) Science 312:1034
Hummer G, Rasaiah JC, Noworyta JP (2001) Nature 414:188
Waghe A, Rasaiah JC, Hummer G (2002) J Chem Phys 117:10789
Maibaum L, Chandler D (2003) J Phys Chem B 107:1189
Ghosh S, Sood AK, Kumar N (2003) Science 299:1042
Koga K, Gao GT, Tanaka H, Zeng XC (2001) Nature 412:802
Ball P (1993) Nature 361:297
Alexiadis A, Kassinos S (2008) Chem Rev 108:5014
Zheng G, Wang Z, Irle S, Morokuma K (2006) J Am Chem Soc 128:15117
Maheshkumar R, Elang M, Subramanian V (2010) J Phys Chem A 114:4313
Lu X, Chen Z (2005) Chem Rev 105:3643
Chandrakumar KRS, Srinivasu K, Ghosh SK (2008) J Phys Chem C 112:15670
Wang L, Zhao J, Li F, Fang H, Lu JP (2009) J Phys Chem C 113:5368
Sharma M, Donadio D, Schwegler E, Galli G (2008) Nano Lett 8:2959
Coudert FX, Vuilleumier R, Boutin A (2006) Chem Phys Chem 7:2464
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunga N, Nguyen KA, Su SJ, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215
Ahlrichs R, Bär M, Häser M, Horn H, Kölmel C (1989) Chem Phys Lett 162:165
Walters EA, Grover JR, White MG, Hui ET (1985) J Phys Chem 89:3814
Levitt M, Perutz MF (1988) J Mol Biol 201:751
Suzuki S, Green PG, Bumgarner RE, Dasgupta S, Goddard WA III, Blake GA (1992) Science 257:942
Rosas I, Alkorta I, Elguero J (1997) J Phys Chem A 101:9457
Prakash M, Gopal SK, Subramanian V (2009) J Phys Chem A 113:13845
Zhao Y, Tishchenko O, Truhlar DG (2005) J Phys Chem B 109:19046
Jenness GR, Jordan KD (2009) J Phys Chem C 113:10242
Franks F (ed) (1972) Water: a comprehensive treatise. Plenum, New York
Rasaiah JC, Garde S, Hummer G (2008) Annu Rev Phys Chem 59:713
Martí J, Gordillo MC (2001) Phys Rev B 63:165430
Feng C, Zhang RQ, Dong SL, Niehaus TA, Frauenheim T (2007) J Phys Chem C 111:14131
Sharma SC, Singh D, Li Y (2005) J Raman Spectrosc 36:755
Kondratyuk P, Yates JT Jr (2007) Acc Chem Res 40:995
Ramachandran CN, Sathyamurthy N (2005) Chem Phys Lett 410:348
Hehre WJ, Radom L, Schleyer PVR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Shameema O, Ramachandran CN, Sathyamurthy N (2006) J Phys Chem A 110:2
Acknowledgments
One of the authors, Naresh K. Jena, gratefully acknowledges Homi Bhabha National Institute (HBNI), Department of Atomic Energy, India, for the award of Senior Research Fellowship. The authors thank Dr. T. Mukherjee for his kind support and the computer center of Bhabha Atomic Research Centre for providing the high-performance parallel computing facilities. The work is also supported by the INDO-EU project MONAMI on computational Materials Science. The support from J. C. Bose fellowship to one of us (Swapan K. Ghosh) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dedicated to Professor Eluvathingal Jemmis and published as part of the special collection of articles celebrating his 60th birthday.
Rights and permissions
About this article
Cite this article
Jena, N.K., Tripathy, M.K., Samanta, A.K. et al. Water molecule encapsulated in carbon nanotube model systems: effect of confinement and curvature. Theor Chem Acc 131, 1205 (2012). https://doi.org/10.1007/s00214-012-1205-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-012-1205-z