Abstract
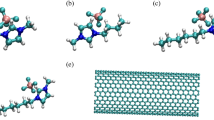
An ab initio investigation on water clusters confined to armchair carbon nanotubes (CNT) with varying diameters has been performed using the density functional theory-based calculations. Different parameters have been investigated including structure, hydrogen bonding pattern and vibrational spectra of water-CNT complexes. Our results reveal that one-dimensional water chain parallel to CNT axis is formed in narrow nanotubes CNT(4,4) and CNT(5,5), whereas in CNT(6,6), zigzag structure is observed. An increase in the CNT diameter results in more symmetric structures similar to the gas phase. The vibrational analysis shows a redshift in stretching frequency of the hydrogen bond assisted O–H in CNT(6,6) due to the reduction in O—O separation whereas a significant blue shift in stretching frequency mode is observed in highly confined CNT(4,4) and CNT(5,5). It implies that the hydrogen bond strength between water molecules is strongest in CNT(6,6). It is also observed that water cluster tends to be near CNT wall due to H···π interaction between water molecule and the π-electron cloud of CNT. An inverse relation between the electronic charge transfer (from CNT to water) and the diameter is also established. This study demonstrates that the degree of confinement is extremely important in deciding the properties of confined water molecules.
Graphic abstract
The structure and hydrogen bonding properties of the water clusters under nano-confined regime is investigated and the results reveal that the intramolecular charge separation for each water molecule increases under confinement and irrespective of the number of water molecules, the highest tube-water interaction energy is achieved in CNT(5,5) (diameter~7 Å). The important finding is that the degree of confinement (diameter of carbon nanotube) is extremely important in deciding the properties of confined water molecules.






Similar content being viewed by others
References
Ball P 2001 In Life’s Matrix: A Biography of Water (Berkeley, California: University of California Press)
Schlichting I, Berendzen J, Chu K, Stock A M, Maves S A, Benson D E, Sweet R M, Ringe D, Petsko G A and Sligar S G 2000 The Catalytic Pathway of Cytochrome P450 cam at Atomic Resolution Science 287 1615
Taraphder S and Hummer G 2003 Protein Side-Chain Motion and Hydration in Proton-Transfer Pathways. Results for Cytochrome P450 cam J. Am. Chem. Soc. 125 3931
de Groot B L 2001 Water Permeation Across Biological Membranes: Mechanism and Dynamics of Aquaporin-1 and GlpF Science 294 2353
Chakraborty S, Kumar H, Dasgupta C and Maiti P K 2017 Confined Water: Structure, Dynamics, and Thermodynamics Acc. Chem. Res. 50 2139
Tripathy M K and Chandrakumar K R S 2017 The exemplary role of nanoconfinement in the proton transfer from acids to ammonia Phys. Chem. Chem. Phys. 19 19869
Bagchi B 2013 In Water In Biological And Chemical Processes: From Structure and Dynamics to Function (Cambridge, UK: Cambridge University Press)
Wolfenden R and Radzicka A 1994 On the probability of finding a water molecule in a nonpolar cavity Science 265 936
Pal S K, Peon J and Zewail A H 2002 Biological water at the protein surface: dynamical solvation probed directly with femtosecond resolution Proc. Natl. Acad. Sci. U.S.A. 99 1763
Ernst J A, Clubb R T, Zhou H X, Gronenborn A M and Clore G M 1995 Demonstration of positionally disordered water within a protein hydrophobic cavity by NMR Science 267 1813
Yu B, Blaber M, Gronenborn A M, Clore G M and Caspar D L 1999 Disordered water within a hydrophobic protein cavity visualized by x-ray crystallography Proc. Natl. Acad. Sci. U.S.A. 96 103
Bernardina S D, Paineau E, Brubach Jean-Blaise, Judeinstein P, Rouzière S, Launois P and Roy P 2016 Water in Carbon Nanotubes: The Peculiar Hydrogen Bond Network Revealed by Infrared Spectroscopy J. Am. Chem. Soc. 138 10437
Holt J K, Park H G, Wang Y, Stadermann M, Artyukhin A B, Grigoropoulos C P, Noy A and Bakajin O 2006 Fast mass transport through sub-2-nanometer carbon nanotubes Science 312 1034
Zhao Y C, Song L, Deng K, Liu Z, Zhang Z X, Yang Y L, Wang C, Yang H F, Jin A Z, Luo Q, Gu C Z, Xie S S and Sun L F 2008 Individual water-filled single-walled carbon nanotubes as hydroelectric power converters Adv. Mater. 20 1772
Yuan Q and Zhao Y P 2009 Hydroelectric voltage generation based on water-filled single-walled carbon nanotubes J. Am. Chem. Soc. 131 6374
Corry B 2008 Designing carbon nanotube membranes for efficient water desalination J. Phys. Chem. B 112 1427
Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A and Kostarelos K 2006 Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers Proc. Natl. Acad. Sci. U.S.A. 103 3357
Koga K, Gao G T, Tanaka H and Zeng X C 2001 Formation of ordered ice nanotubes inside carbon nanotubes Nature 412 802
Maniwa Y, Kataura H, Abe M, Suzuki S, Achiba Y, Kira H and Matsuda K 2002 Phase transition in confined water inside carbon nanotubes J. Phys. Soc. Jpn. 71 2863
Takaiwa D, Hatano I, Koga K and Tanaka H 2008 Phase diagram of water in carbon nanotubes Proc. Natl. Acad. Sci. U.S.A. 105 39
Bai J, Wang J and Zeng X C 2006 Multiwalled ice helixes and ice nanotubes Proc. Natl. Acad. Sci. U.S.A. 103 19664
Koga K, Parra R D, Tanaka H and Zeng X C 2000 Ice nanotube: What does the unit cell look like? J. Chem. Phys. 113 5037
Kyakuno H, Matsuda K, Yahiro H, Inami Y, Fukuoka T, Miyata Y, Yanagi K, Maniwa Y, Kataura H, Saito T, Yumura M and Iijima S 2011 Confined water inside single-walled carbon nanotubes: global phase diagram and effect of finite length J. Chem. Phys. 134 244501
Mann D J and Halls M D 2003 Water alignment and proton conduction inside carbon nanotubes Phys. Rev. Lett. 90 195503
Maniwa Y, Kataura H, Abe M, Udaka A, Suzuki S, Achiba Y, Kira H, Matsuda K, Kadowaki H and Okabe Y 2005 Ordered water inside carbon nanotubes: formation of pentagonal to octagonal ice-nanotubes Chem. Phys. Lett. 401 534
Mikami F, Matsuda K, Kataura H and Maniwa Y 2009 Dielectric properties of water inside single-walled carbon nanotubes ACS Nano 3 1279
Maniwa Y, Matsuda K, Kyakuno H, Ogasawara S, Hibi T, Kadowaki H, Suzuki S, Achiba Y and Kataura H 2007 Water-filled single-wall carbon nanotubes as molecular nanovalves Nat. Mater. 6 135
Shiomi J, Kimura T and Maruyama S 2007 Molecular dynamics of ice-nanotube formation inside carbon nanotubes J. Phys. Chem. C 111 12188
Ghosh S, Ramanathan K V and Sood A K 2004 Water at nanoscale confined in single-walled carbon nanotubes studied by NMR Europhys. Lett. 65 678
Luo C, Fa W, Zhou J, Dong J and Zeng X C 2008 Ferroelectric ordering in ice nanotubes confined in carbon nanotubes Nano Lett. 8 2607
Pascal T A, Goddard W A and Jung Y 2011 Entropy and the driving force for the filling of carbon nanotubes with water Proc. Natl. Acad. Sci. U.S.A. 108 11794
Kumar H, Mukherjee B, Lin S-T, Dasgupta C, Sood A K and Maiti P K 2011 Thermodynamics of water entry in hydrophobic channels of carbon nanotubes J. Chem. Phys. 134 124105
Ghosh S, Sood A K and Kumar N 2003 Carbon nanotube flow sensors Science 299 1042
Lee C Y, Choi W, Han J H and Strano M S 2010 Coherence resonance in a single-walled carbon nanotube ion channel Science 329 1320
Striolo A 2006 The mechanism of water diffusion in narrow carbon nanotubes Nano Lett. 6 633
Walther J H, Ritos K, Cruz-Chu E R, Megaridis C M and Koumoutsakos P 2013 Barriers to superfast water transport in carbon nanotube membranes Nano Lett. 13 1910
Hummer G, Rasaiah J C and Noworyta J P 2001 Water conduction through the hydrophobic channel of a carbon nanotube Nature 414 188
Kolesnikov A I, Zanotti J M, Loong C K, Thiyagarajan P, Moravsky A P, Loutfy R O and Burnham C J 2004 Anomalously soft dynamics of water in a nanotube: a revelation of nanoscale confinement Phys. Rev. Lett. 93 035503
Byl O, Liu J C, Wang Y, Yim W L, Johnson J K and Yates J T Jr. 2006 Unusual hydrogen bonding in water-filled carbon nanotubes J. Am. Chem. Soc. 128 12090
Alexiadis A and Kassinos S 2008 Molecular simulation of water in carbon nanotubes Chem. Rev. 108 5014
Gordillo M C and Marti J 2000 Hydrogen bond structure of liquid water confined in nanotubes Chem. Phys. Lett. 329 341
Mukherjee B, Maiti P K, Dasgupta C and Sood A K 2007 Strong correlations and Fickian water diffusion in narrow carbon nanotubes Strong correlations and Fickian water diffusion in narrow carbon nanotubes J. Chem. Phys. 126 124704
Joseph S and Aluru N R 2008 Why are carbon nanotubes fast transporters of water? Nano Lett. 8 452
Ohba T, Kaneko K, Endo M, Hata K and Kanoh H 2013 Rapid water transportation through narrow one-dimensional channels by restricted hydrogen bonds Langmuir 29 1077
Pérez-Hernández G and Schmidt B 2013 Anisotropy of the water–carbon interaction: molecular simulations of water in low-diameter carbon nanotubes Phys. Chem. Chem. Phys. 15 4995
Thomas J A and McGaughey A J 2008 Reassessing fast water transport through carbon nanotubes Nano Lett. 8 2788
Bankura A and Chandra A 2012 Hydroxide Ion Can Move Faster Than an Excess Proton through One-Dimensional Water Chains in Hydrophobic Narrow Pores J. Phys. Chem. B 116 9744
Bankura A and Chandra A 2015 Proton transfer through hydrogen bonds in two-dimensional water layers: A theoretical study based on ab initio and quantum-classical simulations J. Chem. Phys. 142 44701
Kayal A and Chandra A 2017 Infrared Spectral and Dynamical Properties of Water Confined in Nanobubbles at Hybrid Interfaces of Diamond and Graphene: A Molecular Dynamics Study J. Phys. Chem. C 121 23455
Kayal A and Chandra A 2015 Exploring the structure and dynamics of nano-confined water molecules using molecular dynamics simulations Mol. Simulation 41 463
Agrawal K V, Shimizu S, Drahushuk L W, Kilcoyne D and Strano M S 2017 Observation of extreme phase transition temperatures of water confined inside isolated carbon nanotubes Nat. Nanotechnol. 12 267
Wang J, Zhu Y, Zhou J and Lu X H 2004 Diameter and helicity effects on static properties of water molecules confined in carbon nanotubes Phys. Chem. Chem. Phys. 6 829
Li X, Shi Y, Yang Y, Du H, Zhou R and Zhao Y 2012 How does water-nanotube interaction influence water flow through the nanochannel? J. Chem. Phys. 136 175101
Jena N K, Tripathy M K, Samanta A K, Chandrakumar K R S and Ghosh S K 2012 Water molecule encapsulated in carbon nanotube model systems: Effect of confinement and curvature Theor. Chem. Acc. 131 1205
Hernández-Rojas J, Calvo F, Bretón J and Gomez Llorente J M 2012 Confinement Effects on Water Clusters Inside Carbon Nanotubes J. Phys. Chem. C 116 17019
Ahlrichs R, Bar M, Haser M, Horn H and Kolmel C 1989 Electronic-Structure Calculations on Workstation Computers - the Program System Turbomole Chem. Phys. Lett. 162 165
Bryantsev V S, Diallo M S, van Duin A C and Goddard W A 3rd 2009 Evaluation of B3LYP, X3LYP, and M06-Class Density Functionals for Predicting the Binding Energies of Neutral, Protonated, and Deprotonated Water Clusters J. Chem. Theory Comput. 5 1016
Grimme S 2006 Semiempirical GGA-type density functional constructed with a long-range dispersion correction J. Comput. Chem. 27 1787
Merrick J P, Moran D and Radom L 2007 An evaluation of harmonic vibrational frequency scale factors J. Phys. Chem. A 111 11683
Xantheas S S, Burnham C J and Harrison R J 2002 Development of transferable interaction models for water. III. Reparametrization of an all-atom polarizable rigid model (TTM2–R) from first principles J. Chem. Phys. 116 1493
Xantheas S S and Dunning T H Jr 1993 Ab initio studies of cyclic water clusters (H2O)n, n = 1–6. I. Optimal structures and vibrational spectra J. Chem. Phys. 99 8774
Rowland B, Kadagathur N S, Devlin J P, Buch V, Feldman T and Wojcik M J 1995 Infrared-Spectra of Ice Surfaces and Assignment of Surface-Localized Modes from Simulated Spectra of Cubic Ice J. Chem. Phys. 102 8328
Pribble R N and Zwier T S 1994 Probing hydrogen bonding in benzene–(water)n clusters using resonant ion–dip IR spectroscopy Faraday Discuss. 97 229
Andersson P, Steinbach C and Buck U 2003 Vibrational spectroscopy of large water clusters of known size Eur. Phys. J. D 24 53
Pribble R N and Zwier T S 1994 Size-Specific Infrared Spectra of Benzene-(H2O)n Clusters (n = 1 through 7): Evidence for Noncyclic (H2O)n Structures Science 265 75
Kumar H, Dasgupta C and Maiti P K 2015 Structure, Dynamics and Thermodynamics of Single File water under Confinement: Effects of Polarizability of Water Molecules RSC Adv. 5 1893
Dunn M E, Evans T M, Kirschner K N and Shields G C 2006 Prediction of accurate anharmonic experimental vibrational frequencies for water clusters, (H2O)n, n = 2-5 J. Phys. Chem. A 110 303
Suzuki S, Green P G, Bumgarner R E, Dasgupta S, Goddard W A and Blake G A 1992 Benzene forms hydrogen bonds with water Science 257 942
Rozas I, Alkorta I and Elguero J 1997 Unusual Hydrogen Bonds: H···π Interactions J. Phys. Chem. A 101 9457
Maheshkumar R, Elango M and Subramanian V 2010 Carbohydrate-Aromatic Interactions: The role of curvature on X H··· π Interactions J. Phys. Chem. A 114 4313
Sharma M, Donadio D, Schwegler E and Galli G 2008 Probing properties of water under confinement: Infrared spectra Nano Lett. 8 2959
Acknowledgements
KRSC and MKT gratefully acknowledge the support and encouragement of Dr. T. K. Ghanty and Dr. P. V. Varde and Dr. R. S. Sharma, respectively. The computer Centre of BARC is acknowledged for providing supercomputing facilities. DKM is thankful to IUAC and University of Rajasthan. The fellowship provided by Indian Academy of Sciences through Summer Research Fellowship Programme is gratefully acknowledged by DKM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tripathy, M.K., Mahawar, D.K. & Chandrakumar, K.R.S. Effect of nano-confinement on the structure and properties of water clusters: An ab initio study. J Chem Sci 132, 7 (2020). https://doi.org/10.1007/s12039-019-1697-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-019-1697-3