Abstract
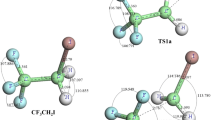
The unimolecular decomposition reaction of CF3CCl2O radical has been investigated using theoretical methods. Two most important channels of decomposition occurring via C–C bond scission and Cl elimination have been considered during the present investigation. Ab initio quantum mechanical calculations are performed to get optimized structure and vibrational frequencies at DFT and MP2 levels of theory. Energetics are further refined by the application of a modified Gaussian-2 method, G2M(CC,MP2). The thermal rate constants for the decomposition reactions involved are evaluated using Canonical Transition State Theory (CTST) utilizing the ab initio data. Rate constants for C–C bond scission and Cl elimination are found to be 6.7 × 106 and 1.1 × 108 s−1, respectively, at 298 K and 1 atm pressure with an energy barrier of 8.6 and 6.5 kcal/mol, respectively. These values suggest that Cl elimination is the dominant process during the decomposition of the CF3CCl2O radical. Transition states are searched on the potential energy surface of the decomposition reactions involved and are characterized by the existence of only one imaginary frequency (NIMAG = 1) during frequency calculation. The existence of transition states on the corresponding potential energy surface is further ascertained by performing intrinsic reaction coordinate (IRC) calculation.





Similar content being viewed by others
References
Solomon S (1990) Nature (Lond) 6291:347–354
Molina MJ, Rowland FS (1974) Nature 249:810–814
Rowland FS, Molina MJ (1994) Chem Eng News 8:72–76
Weubbles DJ (1983) J Geophys Res 88:1433–1443
Scientific Assessment of Stratospheric Ozone (1989) Vol 11; World Meteorological Organization, Global Ozone Research and Monitoring Project Report No. 20
Wayne RP (2001) Chemistry of atmospheres. Clarendon Press, Oxford
Ravishankara AR, Lovejoy ER (1994) J Chem Soc Faraday Trans 90:2159–2169
Geirczak T, Talukdar R, Vaghjiani GL, Lovejoy ER, Ravishankara AR (1991) J Geophys Res 96(D3):5001–5011
Scientific Assessment of Stratospheric Ozone (1995) World Meteorological Organization, Global Ozone Research and Monitoring Project
Atkinson R (1990) Atoms Environ Part A 24A:1–41
Wallington TJ, Hurley MD, Fracheboud JM, Orlando JJ, Tyndall GS, Sehested J, Mogelberg TE, Nielsen OJ (1996) J Phys Chem 100:18116–18122
Brasseur GP, Orlando JJ (1999) Atmospheric chemistry and global change. Oxford University Press, New York
Zellner R (1999) Global aspect of atmospheric chemistry. Steinkopff Darmstadt, Germany
Fuxiang WU, Carr RW (2002) J Phys Chem A 106:5832–5840
Somnitz H, Zellner R (2001) Phys Chem Chem Phys 3:2352–2364
Fuxiang WU, Carr RW (2003) J Phys Chem A 107:10733–10742
Stevens JE, Jabo Khayat RA, Radkevich O, Brown J (2004) J Phys Chem A 108:11354–11361
Hou H, Wang B, Gu Y (2000) J Phys Chem A 104:1570–1575
Hehre WJ, Radom L, Schleyer PvR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr., Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honoda Y, Kitao O, Naki H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VJ, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman GB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, AL-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03; Revision C. 02, Gaussian, Inc, Wallingford
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B37:785–789
Gonzalez C, Schlegel HB (1990) J Chem Phys 94:5523–5527
Curtiss LA, Raghavachari K, Trucks GW, Pople JA (1991) J Chem Phys 94:7221–7230
Mebel AM, Morokuma K, Lin MC (1995) J Chem Phys 103:7414–7421
Frisch A, Nielsen AB, Holder AJ (2000) Gauss view reference. Gaussian Inc, Wallingford
Bhatnagar A, Carr RW (1995) J Phys Chem 99:17573–17577
Bhatnagar A, Carr RW (1996) Chem Phys Lett 258:651–656
Truhlar DG, Garrett BC, Klippenstein SJ (1996) J Phys Chem 100:12771–12800
Wigner EP (1932) Z Phys Chem B19:203–216
Acknowledgments
The authors are thankful to the University Grants Commission, New Delhi for providing fellowships to BKM and NKG under its DSA Program to the Department of Chemistry, DDU Gorakhpur University, Gorakhpur.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, H.J., Mishra, B.K. & Gour, N.K. Theoretical studies of decomposition kinetics of CF3CCl2O radical. Theor Chem Acc 125, 57–64 (2010). https://doi.org/10.1007/s00214-009-0659-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0659-0