Abstract
Rationale
Antidepressant action has been linked to increased synaptic plasticity in which epigenetic mechanisms such as histone posttranslational acetylation could be involved. Interestingly, the histone deacetylases HDAC5 and SIRT2 are oppositely regulated by stress and antidepressants in mice prefrontal cortex (PFC). Besides, the neuroblastoma SH-SY5Y line is an in vitro neuronal model reliable to study drug effects with clear advantages over animals.
Objectives
We aimed to characterize in vitro the role of HDAC5 and SIRT2 in antidepressant regulation of neuroplasticity.
Methods
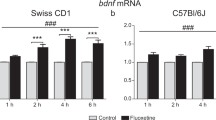
SH-SY5Y cultures were incubated with imipramine, fluoxetine, and reboxetine (10 μM, 2 and 24 h) as well as the selective HDAC5 (MC3822, 5 μM, 24 h) or SIRT2 (33i, 5 μM, 24 h) inhibitors. The regulation of the brain-derived neurotrophic factor (BDNF), the vesicular glutamate transporter 1 (VGLUT1), the acetylated histones 3 (AcH3) and 4 (AcH4), HDAC5, and SIRT2 was studied. Comparatively, the long-term effects of these antidepressants (21 days, i.p.) in the mice (C57BL6, 8 weeks) PFC were studied.
Results
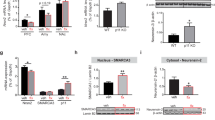
Antidepressants increased both in vitro and in vivo expression of BDNF, VGLUT1, AcH3, and AcH4. Moreover, imipramine and reboxetine increased the phosphorylated form of HDAC5 (P-HDAC5), mediating its cytoplasmic export. Further, SIRT2 was downregulated by all antidepressants. Finally, specific inhibition of HDAC5 and SIRT2 increased neuroplasticity markers.
Conclusions
This study supports the validity of the SH-SY5Y model for studying epigenetic changes linked to synaptic plasticity induced by antidepressants as well as the effect of selective HDAC inhibitors. Particularly, nucleocytoplasmic export of HDAC5 and SIRT2 downregulation mediated by antidepressants could enhance synaptic plasticity markers leading to antidepressant action.









Similar content being viewed by others
References
Abel T, Zukin R (2008) Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol 8:57–64
Alonso R, Griebel G, Pavone G, Stemmelin J, Fur G, Le SP (2004) Blockade of CRF1 or V1b receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry 9:278–286
Ampuero E, Rubio FJ, Falcon R, Sandoval M, Diaz-Veliz G, Gonzalez RE, Earle N, Dagnino-Subiabre A, Aboitiz F, Orrego F, Wyneken U (2010) Chronic fluoxetine treatment induces structural plasticity and selective changes in glutamate receptor subunits in the rat cerebral cortex. Neuroscience 169:98–108
Balschun D, Moechars D, Callaerts-Vegh Z, Vermaercke B, Acker N, Van AL, D’Hooge R (2010) Vesicular glutamate transporter VGLUT1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cereb Cortex 20:684–693
Belzeaux R, Formisano-Tréziny C, Loundou A, Boyer L, Gabert J, Samuelian J-C, Ibrahim EC (2010) Clinical variations modulate patterns of gene expression and define blood biomarkers in major depression. J Psychiatr Res 44(16):1205–1213
Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151
Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS (1978) Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res 38(11 Pt 1):3751–3757
Björkholm C, Monteggia LM (2016) BDNF - a key transducer of antidepressant effects. Neuropharmacology 102:72–79
Bürli RW, Luckhurst CA, Aziz O, Matthews KL, Yates D, Lyons KA, Beconi M, McAllister G, Breccia P, Stott AJ, Penrose SD, Wall M, Lamers M, Leonard P, Müller I, Richardson CM, Jarvis R, Stones L, Hughes S, Wishart G, Haughan AF, O’Connell C, Mead T, McNeil H, Vann J, Mangette J, Maillard M, Beaumont V, Munoz-Sanjuan I, Dominguez C (2013) Design, synthesis, and biological evaluation of potent and selective class IIa histone deacetylase (HDAC) inhibitors as a potential therapy for Huntington’s disease. J Med Chem 56:9934–9954
Chawla S, Vanhoutte P, Arnold FJL, Huang CL-H, Bading H (2003) Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem 85:151–159
Choi M, Lee SH, Wang SE, Ko SY, Song M, Choi J-S, Kim Y-S, Duman RS, Son H (2015) Ketamine produces antidepressant-like effects through phosphorylation-dependent nuclear export of histone deacetylase 5 (HDAC5) in rats. Proc Natl Acad Sci U S A 112:15755–15760
Cooke JD, Cavender HM, Lima HK, Grover LM (2014) Antidepressants that inhibit both serotonin and norepinephrine reuptake impair long-term potentiation in hippocampus. Psychopharmacology 231:4429–4441
Covington HE, Vialou VF, LaPlant Q, Ohnishi YN, Nestler EJ (2011) Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci Lett 493:122–126
Czéh B, Müller-Keuker JIH, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E (2007) Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology 32:1490–1503
Donnici L, Tiraboschi E, Tardito D, Musazzi L, Racagni G, Popoli M (2008) Time-dependent biphasic modulation of human BDNF by antidepressants in neuroblastoma cells. BMC Neurosci 9:61
Dulac C (2010) Brain function and chromatin plasticity. Nature 465:728–735
Duman CH, Duman RS (2015) Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett 601:20–29
Duman RS (2002) Synaptic plasticity and mood disorders. Mol Psychiatry 7:S29–S34
Duman RS (2014) Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depress Anxiety 31:291–296
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127
Erburu M, Muñoz-Cobo I, Diaz-Perdigon T, Mellini P, Suzuki T, Puerta E, Tordera RM (2017) SIRT2 inhibition modulate glutamate and serotonin systems in the prefrontal cortex and induces antidepressant-like action. Neuropharmacology 117:195–208
Erburu M, Muñoz-Cobo I, Domínguez-Andrés J, Beltran E, Suzuki T, Mai A, Valente S, Puerta E, Tordera RM (2015) Chronic stress and antidepressant induced changes in Hdac5 and Sirt2 affect synaptic plasticity. Eur Neuropsychopharmacol 25:2036–2048
Eskandarian HA, Impens F, Nahori M-A, Soubigou G, Coppée J-Y, Cossart P, Hamon MA (2013) A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science 341:1238858
Farley S, Dumas S, Mestikawy S, El GB (2012) Increased expression of the vesicular glutamate transporter-1 (VGLUT1) in the prefrontal cortex correlates with differential vulnerability to chronic stress in various mouse strains: effects of fluoxetine and MK-801. Neuropharmacology 62:503–517
Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH (2001) The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31:247–260
Gallinari P, Marco S, Di JP, Pallaoro M, Steinkühler C (2007) HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res 17:195–211
Garcia-Garcia AL, Elizalde N, Matrov D, Harro J, Wojcik SM, Venzala E, Ramírez MJ, Rio J, Del TRM (2009) Increased vulnerability to depressive-like behavior of mice with decreased expression of VGLUT1. Biol Psychiatry 66:275–282
Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, EL MS (2002) A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci 22:5442–5451
Guedes-Dias P, Oliveira JMA (2013) Lysine deacetylases and mitochondrial dynamics in neurodegeneration. Biochim Biophys Acta - Mol Basis Dis 1832:1345–1359
Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C et al (2001) The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neuroscience : Official J Soc Neuroscience 21(22):RC181
Hobara T, Uchida S, Otsuki K, Matsubara T, Funato H, Matsuo K, Suetsugi M, Watanabe Y (2010) Altered gene expression of histone deacetylases in mood disorder patients. J Psychiatr Res 44:263–270
Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR (2000) Comparative Cytoarchitectonic Atlas of the C57BL6 and 129Sv Mouse Brains, (Elsevier, ed). Elsevier, New Yorrk
Lindholm JS, Castrén E (2014) Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Front Behav Neurosci 8:143
Machado-Vieira R, Salvadore G, DiazGranados N, Ibrahim L, Latov D, Wheeler-Castillo C, Zarate CA (2010) New therapeutic targets for mood disorders. Sci World J 10:713–726
Mangas-Sanjuan V, Oláh J, Gonzalez-Alvarez I, Lehotzky A, Tőkési N, Bermejo M, Ovádi J (2015) Tubulin acetylation promoting potency and absorption efficacy of deacetylase inhibitors. Br J Pharmacol 172:829–840
Muñoz-Cobo I, Belloch FB, Díaz-Perdigón T, Puerta E, Tordera RM (2017) SIRT2 inhibition reverses anhedonia in the VGLUT1+/− depression model. Behav Brain Res 335:128–131. https://doi.org/10.1016/j.bbr.2017.07.045
McKinsey TA, Zhang CL, Olson EN (2001) Identification of a signal-responsive nuclear export sequence in class II histone deacetylases. Mol Cell Biol 21:6312–6321
Moutsimilli L, Farley S, Dumas S, El Mestikawy S, Giros B, Tzavara ET (2005) Selective cortical VGLUT1 increase as a marker for antidepressant activity. Neuropharmacology 54:497–508
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002) Neurobiology of depression. Neuron 34:13–25
Outeiro, T. F., Kontopoulos, E., Altmann, S. M., Kufareva, I., Strathearn, K. E., Amore, A. M., & Kazantsev, A. G. (2007). Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science (New York, N.Y.), 317(5837), 516–9
Pandithage R, Lilischkis R, Harting K, Wolf A, Jedamzik B, Lüscher-Firzlaff J, Lüscher B (2008) The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol 180(5):915–929
Phillips C (2017) Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plast. 2017:7260130
Porcelli S, Salfi R, Politis A, Atti AR, Albani D, Chierchia A, Serretti A (2013) Association between Sirtuin 2 gene rs10410544 polymorphism and depression in Alzheimer’s disease in two independent European samples. J Neural Transmission (Vienna, Austria : 1996) 120(12):1709–1715
Parra M, Verdin E (2010) Regulatory signal transduction pathways for class IIa histone deacetylases. Curr Opin Pharmacol 10:454–460
Renthal W, Maze I, Krishnan V, Covington HE, Xiao G, Kumar A, Russo SJ et al (2007) Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 56:517–529
Réus GZ, Abelaira HM, Santos MAB, dos Carlessi AS, Tomaz DB, Neotti MV, Liranço JLG et al (2013) Ketamine and imipramine in the nucleus accumbens regulate histone deacetylation induced by maternal deprivation and are critical for associated behaviors. Behav Brain Res 256:451–456
Ross RA, Spengler BA, Biedler JL (1983) Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst 71(4):741–747
Rumpf T, Schiedel M, Karaman B, Roessler C, North BJ, Lehotzky A, Oláh J, Ladwein KI, Schmidtkunz K, Gajer M, Pannek M, Steegborn C, Sinclair DA, Gerhardt S, Ovádi J, Schutkowski M, Sippl W, Einsle O, Jung M (2015) Selective Sirt2 inhibition by ligand-induced rearrangement of the active site. Nat Commun 6:6263
Sacchetti G, Bernini M, Bianchetti A, Parini S, Invernizzi RW, Samanin R (1999) Studies on the acute and chronic effects of reboxetine on extracellular noradrenaline and other monoamines in the rat brain. Br J Pharmacol 128:1332–1338
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9:671–675
Shadfar S., Kim Y.-G., Katila N., Neupane S., Ojha U., Bhurtel S., Srivastav S., et al. (2016) Neuroprotective effects of antidepressants via upregulation of neurotrophic factors in the MPTP model of Parkinson’s disease. Mol Neurobiol
Southwick SM, Vythilingam M, Charney DS (2005) The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol 1:255–291
Sucharov CC, Dockstader K, Nunley K, McKinsey TA, Bristow M (2011) β-Adrenergic receptor stimulation and activation of protein kinase a protect against α1-adrenergic-mediated phosphorylation of protein kinase D and histone deacetylase 5. J Card Fail 17:592–600
Suzuki T, Khan MNA, Sawada H, Imai E, Itoh Y, Yamatsuta K, Tokuda N, Takeuchi J, Seko T, Nakagawa H, Miyata N (2012) Design, synthesis, and biological activity of a novel series of human Sirtuin-2-selective inhibitors. J Med Chem 55:5760–5773
Tordera RM, Monge A, Del Río J, Lasheras B (2002) Antidepressant-like activity of VN2222, a serotonin reuptake inhibitor with high affinity at 5-HT1A receptors. Eur J Pharmacol. 442:63–71
Tordera RM, Pei Q, Sharp T (2005) Evidence for increased expression of the vesicular glutamate transporter, VGLUT1, by a course of antidepressant treatment. J Neurochem 94:875–883
Tordera RM, Totterdell S, Wojcik SM, Brose N, Elizalde N, Lasheras B, Del RJ (2007) Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1). Eur J Neurosci 25:281–290
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006) Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–525
Uezato A, Meador-Woodruff JH, McCullumsmith RE (2009) Vesicular glutamate transporter mRNA expression in the medial temporal lobe in major depressive disorder, bipolar disorder, and schizophrenia. Bipolar Disord 11:711–725
Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA (2004) Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol 24:8374–8385
Villanueva R (2013) Neurobiology of major depressive disorder. Neural Plast 2013:1–7
Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C (2004) An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci 101:7158–7163
Acknowledgments
We are very grateful to Ms. Sandra Lizaso and Mr. Mikel Aleixo for their excellent technical assistance. We also thank Prof. T. Suzuki from Kyoto Prefectural University of Medicine, Japan, for the kind donation of the compound 33i. We are also very grateful to Dr. Mikel Ariz and Ms. Ainhoa Urbiola from the Imaging Platform of the Center for Applied Medical Research (CIMA).
Funding
This work was supported by the Ministry of Economy and Competitiveness (SAF2011-27910, Spanish Government) (Dr. R. Tordera) and the JST CREST program (Dr T. Suzuki). Moreover, PhD students were supported by fellowship from the Spanish Ministry of Economy and Competitiveness (SAF2011-27910) to I. Muñoz-Cobo and from the “Asociación de Amigos de la Universidad de Navarra, Spain” to M. Erburu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experimental procedures and animal husbandry were conducted according to the principles of laboratory animal care as detailed in the European Communities Council Directive (2013/53/EC) and approved by the Ethical Committee of University of Navarra.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Muñoz-Cobo, I., Erburu, M., Zwergel, C. et al. Nucleocytoplasmic export of HDAC5 and SIRT2 downregulation: two epigenetic mechanisms by which antidepressants enhance synaptic plasticity markers. Psychopharmacology 235, 2831–2846 (2018). https://doi.org/10.1007/s00213-018-4975-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4975-8