Abstract
Rationale
There is a significant interest in the NMDA-receptor antagonist ketamine due to its efficacy in treating depressive disorders and its induction of psychotic-like symptoms that make it a useful tool for modeling psychosis. Pharmacological MRI in awake nonhuman primates provides a highly translational model for studying the brain network dynamics involved in producing these drug effects.
Objective
The present study evaluated ketamine-induced changes in functional connectivity (FC) in awake rhesus monkeys. The effects of ketamine after pretreatment with the antipsychotic drug risperidone were also examined.
Methods
Functional MRI scans were conducted in four awake adult female rhesus monkeys during sub-anesthetic i.v. infusions of ketamine (0.345 mg/kg bolus followed by 0.256 mg kg−1 h−1 constant infusion) with and without risperidone pretreatment (0.06 mg/kg). A 10-min window of stable BOLD signal was used to compare FC between baseline and drug conditions. FC was assessed in specific regions of interest using seed-based cross-correlation analysis.
Results
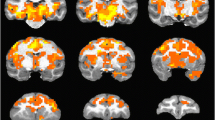
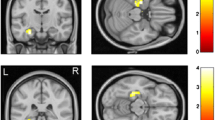
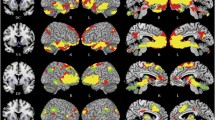
Ketamine infusion induced extensive changes in FC. In particular, FC to the dorsolateral prefrontal cortex (dlPFC) was increased in several cortical and subcortical regions. Pretreatment with risperidone largely attenuated ketamine-induced changes in FC.
Conclusions
The results are highly consistent with similar human imaging studies showing ketamine-induced changes in FC, as well as a significant attenuation of these changes when ketamine infusion is preceded by pretreatment with risperidone. The extensive increases shown in FC to the dlPFC are consistent with the idea that disinhibition of the dlPFC may be a key driver of the antidepressant and psychotomimetic effects of ketamine.






Similar content being viewed by others
References
Adachi Y, Osada T, Sporns O, Watanabe T, Matsui T, Miyamoto K, Miyashita Y (2012) Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex 22:1586–1592
Agid O, Kapur S, Arenovich T, Zipursky RB (2003) Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 60:1228–1235
Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M (2009) Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res 171:189–198
Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, Krystal JH, Pearlson GD, Glahn DC (2013) Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry 73:565–573
Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y, Repovs G, Murray JD, Driesen NR, Morgan PT, Xu K, Wang F, Krystal JH (2015) N-methyl-d-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry 77:569–580
Arnsten AF, Wang MJ, Paspalas CD (2012) Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76:223–239
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354
Catafau AM, Corripio I, Perez V, Martin JC, Schotte A, Carrio I, Alvarez E (2006) Dopamine D2 receptor occupancy by risperidone: implications for the timing and magnitude of clinical response. Psychiatry Res 148:175–183
Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, Cohen BM, Ongur D (2011) Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology 36:2009–2017
Cohen SM, Tsien RW, Goff DC, Halassa MM (2015) The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res 167:98–107
Concerto C, Lanza G, Cantone M, Ferri R, Pennisi G, Bella R, Aguglia E (2015) Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: a six-month clinical follow-up study. Int J Psychiatry Clin Pract 19:252–258
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173
Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T (2001) Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Brain Res Rev 37:153–161
Dandash O, Harrison BJ, Adapa R, Gaillard R, Giorlando F, Wood SJ, Fletcher PC, Fornito A (2015) Selective augmentation of striatal functional connectivity following NMDA receptor antagonism: implications for psychosis. Neuropsychopharmacology 40:622–631
De Simoni S, Schwarz AJ, O’Daly OG, Marquand AF, Brittain C, Gonzales C, Stephenson S, Williams SC, Mehta MA (2013) Test-retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. NeuroImage 64:75–90
Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM (2008) Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry 65:154–164
Doyle OM, De Simoni S, Schwarz AJ, Brittain C, O’Daly OG, Williams SC, Mehta MA (2013) Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: a validation using antipsychotic and glutamatergic agents. J Pharmacol Exp Ther 345:151–160
Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, Anticevic A, Morgan PT, Krystal JH (2013b) Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry 18:1199–1204
Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D’Souza DC, Gueorguieva R, He G, Leung HC, Ramani R, Anticevic A, Suckow RF, Morgan PT, Krystal JH (2013a) The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology 38:2613–2622
Dutta A, McKie S, Deakin JF (2014) Resting state networks in major depressive disorder. Psychiatry Res 224:139–151
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995) Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33:636–647
Frangou S (2014) A systems neuroscience perspective of schizophrenia and bipolar disorder. Schizophr Bull 40:523–531
Frangou S, Kington J, Raymont V, Shergill SS (2008) Examining ventral and dorsal prefrontal function in bipolar disorder: a functional magnetic resonance imaging study. Eur Psychiatry 23:300–308
Frohlich J, Van Horn JD (2014) Reviewing the ketamine model for schizophrenia. J Psychopharmacol 28:287–302
Goya-Maldonado R, Brodmann K, Keil M, Trost S, Dechent P, Gruber O (2016) Differentiating unipolar and bipolar depression by alterations in large-scale brain networks. Hum Brain Mapp 37:808–818
Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007) Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437
Grimm O, Gass N, Weber-Fahr W, Sartorius A, Schenker E, Spedding M, Risterucci C, Schweiger JI, Bohringer A, Zang Z, Tost H, Schwarz AJ, Meyer-Lindenberg A (2015) Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology 232:4231–4241
Guo S, Kendrick KM, Zhang J, Broome M, Yu R, Liu Z, Feng J (2013) Brain-wide functional inter-hemispheric disconnection is a potential biomarker for schizophrenia and distinguishes it from depression. NeuroImage Clin 2:818–826
Hadley JA, Nenert R, Kraguljac NV, Bolding MS, White DM, Skidmore FM, Visscher KM, Lahti AC (2014) Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology 39:1020–1030
Hutchison RM, Gallivan JP, Culham JC, Gati JS, Menon RS, Everling S (2012) Functional connectivity of the frontal eye fields in humans and macaque monkeys investigated with resting-state fMRI. J Neurophysiol 107:2463–2474
Joules R, Doyle OM, Schwarz AJ, O’Daly OG, Brammer M, Williams SC, Mehta, MA (2015) Ketamine induces a robust whole-brain connectivity pattern that can be differentially modulated by drugs of different mechanism and clinical profile. Psychopharmacol (Berl)
Khalili-Mahani N, Niesters M, van Osch MJ, Oitzl M, Veer I, de Rooij M, van Gerven J, van Buchem MA, Beckmann CF, Rombouts SA, Dahan A (2015) Ketamine interactions with biomarkers of stress: a randomized placebo-controlled repeated measures resting-state fMRI and PCASL pilot study in healthy men. NeuroImage 108:396–409
Kraguljac NV, White DM, Hadley JA, Visscher K, Knight D, Ver Hoef L, Falola B, Lahti AC (2016) Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. NeuroImage Clin 10:146–158
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Krystal JH, Sanacora G, Duman RS (2013) Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73:1133–1141
Lewis DA, Lieberman JA (2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28:325–334
Maltbie E, Gopinath K, Urushino N, Kempf D, Howell L (2016) Ketamine-induced brain activation in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology 233:961–972
Mayberg HS (2003) Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65:193–207
Meyer-Lindenberg A (2010) From maps to mechanisms through neuroimaging of schizophrenia. Nature 468:194–202
Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF (2005) Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62:379–386
Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009) Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66:811–822
Miyamoto S, Duncan GE, Marx CE, Lieberman JA (2005) Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10:79–104
Muly EC, Votaw JR, Ritchie J, Howell LL (2012) Relationship between dose, drug levels, and D2 receptor occupancy for the atypical antipsychotics risperidone and paliperidone. J Pharmacol Exp Ther 341:81–89
Murnane KS, Gopinath KS, Maltbie E, Daunais JB, Telesford QK, Howell LL (2015) Functional connectivity in frontal-striatal brain networks and cocaine self-administration in female rhesus monkeys. Psychopharmacology 232:745–754
Murnane KS, Howell LL (2010) Development of an apparatus and methodology for conducting functional magnetic resonance imaging (fMRI) with pharmacological stimuli in conscious rhesus monkeys. J Neurosci Methods 191:11–20
Nyberg S, Farde L, Eriksson L, Halldin C, Eriksson B (1993) 5-HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology 110:265–272
Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF (2010) Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res 183:59–68
Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML (2014) Why primate models matter. Am J Primatol 76:801–827
Phillips ML, Swartz HA (2014) A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry 171:829–843
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154
Preuss TM (1995) Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci 7:1–24
Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, Pfefferbaum A (2012) The INIA19 template and NeuroMaps Atlas for primate brain image parcellation and spatial normalization. Front Neuroinflammation 6:27
Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE (2010) Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res 117:21–30
Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012) Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32
Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, Gallego JA, Kane JM, Szeszko PR, Malhotra AK (2015) Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 72:5–13
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):S208–S219
van den Buuse M, Mingon RL, Gogos A (2015) Chronic estrogen and progesterone treatment inhibits ketamine-induced disruption of prepulse inhibition in rats. Neurosci Lett 607: 72–6.
Wang M, Arnsten AF (2015) Contribution of NMDA receptors to dorsolateral prefrontal cortical networks in primates. Neurosci Bull 31:191–197
Wessa M, Kanske P, Linke J (2014) Bipolar disorder: a neural network perspective on a disorder of emotion and motivation. Restor Neurol Neurosci 32:51–62
Woodward ND, Rogers B, Heckers S (2011) Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res 130:86–93
Zhang J, Kendrick KM, Lu G, Feng J (2015) The fault lies on the other side: altered brain functional connectivity in psychiatric disorders is mainly caused by counterpart regions in the opposite hemisphere. Cereb Cortex 25:3475–3486
Acknowledgments
The authors declare no competing financial interests. This research was supported by P51OD11132 (Yerkes National Primate Research Center), Sunovion Pharmaceutical, Ltd. (LLH) and DA031246 (LLH). Special thanks to the Yerkes Imaging Center, technicians Marisa Olsen and Juliet Brown, and Christopher Muly, MD, PhD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kaundinya Gopinath and Eric Maltbie contributed equally to this work.
Electronic supplementary material
ESM 1
(DOCX 6898 kb)
Rights and permissions
About this article
Cite this article
Gopinath, K., Maltbie, E., Urushino, N. et al. Ketamine-induced changes in connectivity of functional brain networks in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology 233, 3673–3684 (2016). https://doi.org/10.1007/s00213-016-4401-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4401-z