Abstract
Rationale
Social defeat stress induces physiological and behavioral symptoms, including anxiety, anhedonia, immune deficits, and altered expression of key brain genes.
Objectives
The present study investigated the effects of social defeat stress on the behaviors and expressions of Chat, Grp78, and chop in the brains of adult mice.
Methods
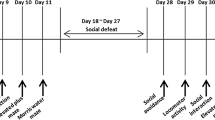
Adult mice were divided into susceptible and unsusceptible groups after 10 days of social defeat stress. In experiment 1, behavioral tests were conducted, and brains were processed for Western blotting at day 27 after stress. In experiment 2, social avoidance tests were conducted, and brains were processed for Western blotting at day 12 after stress.
Results
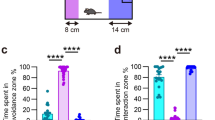
The results indicate decreased and increased locomotion and anxiety behavior in all defeated mice. Decrease in social interaction, increased immobility, and impaired memory performance were only observed in susceptible mice. A decrease in the Chat level at days 12 and 27 was noted in the prefrontal cortex (PFC), amygdala (Amyg), and dorsal hippocampus (HIP) in defeated mice. The expression levels of Grp78 and chop measured on days 12 and 27 were significantly greater in the Amyg of susceptible mice. In the PFC and HIP, defeated mice displayed different patterns in the levels of Grp78 and chop expressions measured on days 12 and 27.
Conclusions
The present study demonstrated that chronic social defeat stress in mice produces stress-related behaviors. Different response patterns were noted for Grp78 and chop expression among the groups in terms of brain regions and time-course effects.










Similar content being viewed by others
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. Washington, DC: American Psychiatric Association
Aubert I, Rowe W, Meaney MJ, Gauthier S, Quirion R (1995) Cholinergic markers in aged cognitively impaired Long–Evans rats. Neuroscience 67(2):277–292
Bertaina-Anglade V, Enjuanes E, Morillon D, Drieu la Rochelle C (2006) The object recognition task in rats and mice: a simple and rapid model in safety pharmacology to detect amnesic properties of a new chemical entity. J Pharmacol Toxicol Methods 54:99–105
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ et al (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868
Bown C, Wang JF, MacQueen G, Young LT (2000) Increased temporal cortex ER stress proteins in depressed subjects who died by suicide. Neuropsychopharmacology 22(3):327–332
Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM et al (2005) Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev 29(1):83–97
Colombo PJ, Gallagher M (1998) Individual differences in spatial memory and striatal ChAT activity among young and aged rats. Neurobiol Learn Mem 70(3):314–327
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36:60–90
El Hage W, Peronny S, Griebel G, Belzung C (2004) Impaired memory following predatory stress in mice is improved by fluoxetine. Prog Neuropsychopharmacol Biol Psychiatry 28:123–128
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. I: behavioral data. Behav Brain Res 31:47–59
Fontanier-Razzaq NC, Hay SM, Rees WD (1999) Upregulation of CHOP-10 (gadd153) expression in the mouse blastocyst as a response to stress. Mol Reprod Dev 54:326–332
Gottesfeld Z, Kvetňanskŷ KIJ, Jacobowitz DM (1978) Effects of repeated immobilization stress on glutamate decarboxylase and choline acetyltransferase in discrete brain regions. Brain Res 152:374–378
Haubrich D, Chippendale T (1977) Regulation of acetylcholine synthesis in nervous tissue. Life Sci 20(9):1465–1478
Ishisaka M, Kudo T, Shimazawa M, Kakefuda K, Oyagi A, Hyakkoku K et al (2011) Restraint-induced expression of endoplasmic reticulum stress-related genes in the mouse brain. Pharmacology & Pharmacy 2:10–16
Jenden D, Jope R, Weiler M (1976) Regulation of acetylcholine synthesis: does cytoplasmic acetylcholine control high affinity choline uptake? Science 194(4265):635–637
Kessler RC, Price RH, Wortman CB (1985) Social factors in psychopathology: stress, social support, and coping processes. Annu Rev Psychol 36:531–572
Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R (2007) Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav Immun 21:458–466
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ et al (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404
Lee AS (2001) The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 26(8):504–510
Malatynska E, Knapp RJ (2005) Dominant–submissive behavior as model of mania and depression. Neurosci Biobehav Rev 29:715–737
Maren S, Quirk GJ (2004) Neuronal signaling of fear memory. Nature 5:844–852
Maytin EV, Ubeda M, Lin JC, Habener JF (2001) Stress-inducible transcription factor CHOP/gadd153 induces apoptosis in mammalian cells via p38 kinase-dependent and -independent mechanisms. Exp Cell Res 267:193–204
Naidoo N, Giang W, Galante RJ, Pack AI (2005) Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem 92:1150–1157
Nestler EJ, Carlezon WA (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159
Oda Y (1999) Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int 49:921–937
Park HJ, Shim HS, Kim KS, Lee HL, Hahm DH, Shim I (2010) Effects of glycyrrhizae radix on repeated restraint stress-induced neurochemical and behavioral responses. Korean J Physiol Pharmacol 14:371–376
Razzoli M, Carboni L, Andreoli M, Ballottari A, Arban R (2010) Different susceptibility to social stress of BalbC and C57BL6/J mice. Behav Brain Res 216(1):100–108
Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M (2010) Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry 15(12):1152–1163
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006) Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–525
von Frijtag JC, Reijmers LG, van der Harst JE, Leus IE, Van den BR, Spruijt BM (2000) Defeat followed by individual hosing results in long-term impaired reward- and cognition-related behaviors in rats. Behav Brain Res 117:137–146
Wahba ZZ, Soliman KFA (1992) Effect of stress on choline acetyltransferase activity of the brain and the adrenal of the rat. Experientia 48:265–268
West AP (1990) Neurobehavioral studies of forced swimming: the role of learning and memory in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry 14:863–877
Wu D, Hersh LB (1994) Choline acetyltransferase: celebrating its fiftieth year. J Neurochem 62:1653–1663
Yu T, Guo M, Garza J, Rendon S, Sun XL, Zhang W et al (2011) Cognitive and neural correlates of depression-like behavior in socially defeated mice: an animal model of depression with cognitive dysfunction. Int J Neuropsychopharmacol 14(3):303–317
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (2012-0007635).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, T., Huang, GB., Muna, S.S. et al. Effects of chronic social defeat stress on behavior and choline acetyltransferase, 78-kDa glucose-regulated protein, and CCAAT/enhancer-binding protein (C/EBP) homologous protein in adult mice. Psychopharmacology 228, 217–230 (2013). https://doi.org/10.1007/s00213-013-3028-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3028-6