Abstract
Rationale
Among other monoamine neurotransmitters, dopamine is implicated in the pathophysiology of major depression. Experimental studies suggest the involvement of the mesolimbic dopamine system in the mechanism of action of antidepressant drugs. Previous in vivo imaging studies have studied striatal dopamine D2 receptor availability in depression but the results are equivocal thus far.
Objective
To study the striatal and thalamic dopamine D2 receptor availability in drug-naive patients with major depression was the aim of this study.
Materials and methods
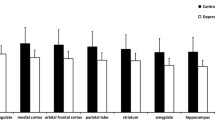
Caudate, putamen, and thalamic dopamine D2 receptor availability was estimated using positron emission tomography and [11C]raclopride in 25 treatment-seeking drug-free patients (of whom 24 were drug-naive) with major depression (primary care patients) as well as in 19 demographically similar healthy control subjects. Receptor availability was expressed as the binding potential (BPND), and analyses were carried out based on both regional and voxel-level BPND estimates.
Results
No statistically significant differences in [11C]raclopride BPND were observed between the groups either in the caudate nucleus (+1.7%, CI −4.8% to +8.3%), putamen (−1.0%, CI −7.2% to 5.1%), thalamus (−2.4%, CI −8.7% to 4.0%), or ventral striatum (−3.8%, CI −9.3% to +1.6%). In the patients, depressive symptoms were not associated with [11C]raclopride BPND in any region.
Conclusions
The findings in this sample of treatment-seeking, drug-naive and predominantly first-episode patients with major depression do not support the involvement of striatal dopamine D2 receptors in the pathophysiology of the illness, but do not exclude the potential importance of dopaminergic mechanisms in antidepressant drug action.


Similar content being viewed by others
References
Ågren H, Reibring L, Hartvig P, Tedroff J, Bjurling P, Lundqvist H, Långström B (1993) Monoamine metabolism in human prefrontal cortex and basal ganglia. PET studies using [beta-11C]1-5-hydroxytryptophan and [beta-11C]L-dopa in healthy volunteers and patients with unipolar major depression. Depression 1:71–81
Allard P, Norlen M (2001) Caudate nucleus dopamine D2 receptors in depressed suicide victims. Neuropsychobiology 44:70–73
Argyelan M, Szabo Z, Kanyo B, Tanacs A, Kovacs Z, Janka Z, Pavics L (2005) Dopamine transporter availability in medication free and in bupropion treated depression: a 99mTc-TRODAT-1 SPECT study. J Affect Disord 89:115–123
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–71
Bowden C, Theodorou AE, Cheetham SC, Lowther S, Katona CL, Crompton MR, Horton RW (1997) Dopamine D1 and D2 receptor binding sites in brain samples from depressed suicides and controls. Brain Res 752:227–233
Brunswick DJ, Amsterdam JD, Mozley PD, Newberg A (2003) Greater availability of brain dopamine transporters in major depression shown by [99mTc]TRODAT-1 SPECT imaging. Am J Psychiatry 160:1836–1841
D’Aquila PS, Collu M, Gessa GL, Serra G (2000) The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol 405:365–373
D’haenen HA, Bossuyt A (1994) Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biol Psychiatry 35:28–32
Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA (2001) Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry 49:81–96
Dunlop BW, Nemeroff CB (2007) The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64:327–337
Dupont WD, Plummer WD (1990) Power and sample size calculations: a review and computer program control. Control Clin Trials 11:116–128
Ebert D, Feistel H, Loew T, Pirner A (1996) Dopamine and depression—striatal dopamine D2 receptor SPECT before and after antidepressant therapy. Psychopharmacology 126:91–94
Finnema SJ, Seneca N, Farde L, Shchulkin E, Sóvágó J, Gulyás B, Wikström JV, Innis RB, Neumeyer JL, Halldin C (2005) A preliminary PET evaluation of the new dopamine D2 receptor agonist [11C]MNPA in cynomolgus monkey. Nucl Med Biol 32:353–360
First MB, Spitzer RL, Gibbon M, Williams JB (1997) Structured clinical interview for DSM-Axis I disorders—clinician version (SCID-CV). American Psychiatric, Washington, DC
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith C, Frackowiak RSJ (1995) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210
Gershon AA, Vishne T, Grunhaus L (2007) Dopamine D2-like receptors and the antidepressant response. Biol Psychiatry 61:145–153
Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ (1997) Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 6:279–287
Hamilton M (1967) Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6:278–296
Hirvonen J, Aalto S, Lumme V, Någren K, Kajander J, Vilkman H, Hagelberg N, Oikonen V, Hia J et al (2003) Measurement of striatal and thalamic dopamine D2 receptor binding with [11C]raclopride. Nucl Med Commun 24:1207–1214
Hirvonen J, van Erp TGM, Huttunen J, Aalto S, Någren K, Huttunen M, Lönnqvist J, Kaprio J, Hia J, Cannon TD et al (2005) Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Arch Gen Psychiatry 62:371–378
Hirvonen J, van Erp TG, Huttunen J, Någren K, Huttunen M, Aalto S, Lönnqvist J, Kaprio J, Cannon TD, Hia J et al (2006) Striatal dopamine D1 and D2 receptor balance in twins at increased genetic risk for schizophrenia. Psychiatry Res Neuroimaging 146:13–20
Hwang DR, Narendran R, Laruelle M (2005) Positron-labeled dopamine agonists for probing the high affinity states of dopamine subtype 2 receptors. Bioconjug Chem 16:27–31
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539 May
Joel D, Weiner I (2000) The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96:451–474
Kapur S, Mann JJ (1992) Role of the dopaminergic system in depression. Biol Psychiatry 32:1–17
Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA (2002) Dopaminergic abnormalities in amygdaloid nuclei in major depression: a post-mortem study. Biol Psychiatry 52:740–748
Klimke A, Larisch R, Janz A, Vosberg H, Müller-Gärtner HW, Gaebel W (1999) Dopamine D2 receptor binding before and after treatment of major depression measured by [123I]IBZM SPECT. Psychiatry Res 90:91–101
Kuroda Y, Motohashi N, Ito H, Ito S, Takano A, Nishikawa T, Suhara T (2006) Effects of repetitive transcranial magnetic stimulation on [11C]raclopride binding and cognitive function in patients with depression. J Affect Disord 95:35–42
Laasonen-Balk T, Kuikka J, Viinamäki H, Husso-Saastamoinen M, Lehtonen J, Tiihonen J (1999) Striatal dopamine transporter density in major depression. Psychopharmacology 144:282–285
Lammertsma AA, Hume SP (1996) Simplified reference tissue model for PET receptor studies. Neuroimage 4:153–158
Laruelle M (2000) Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 203:423–451
Malhi GS, Berk M (2007) Does dopamine dysfunction drive depression. Acta Psychiatr Scand 115(Suppl 433):116–124
Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS (1998) Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 44:1090–1098
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M (2001) Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21:1034–1057
Meyer JH, Gunn RN, Myers R, Grasby PM (1999) Assessment of spatial normalization of PET ligand images using ligand-specific templates. Neuroimage 9:545–553
Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff NPLG, Wilson AA, Houle S (2006) Elevated putamen D2 receptor binding potential in major depression with motor retardation: an [11C]raclopride positron emission tomography study. Am J Psychiatry 163:1594–1602
Montgomery AJ, Stokes P, Kitamura Y, Grasby PM (2007) Extrastriatal D2 and striatal D2 receptor in depressive illness: pilot PET studies using [11C]FLB 457 and [11C]raclopride. J Affect Disord 101:113–122
Nestler EJ, Carlezon WA Jr. (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159
Nieoullon A (2002) Dopamine and the regulation of cognition and attention. Prog Neurobiol 67:53–83
Paillere-Martinot ML, Bragulat V, Artiges E, Dolle F, Hinnen F, Jouvent R, Martinot JL (2001) Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am J Psychiatry 158:314–316
Parsey RV, Oquendo MA, Zea-Ponce Y, Rodenhiser J, Kegeles LS, Pratap M, Cooper TB, Van Heertum R, Mann JJ, Laruelle M (2001) Dopamine D2 receptor availability and amphetamine-induced dopamine release in unipolar depression. Biol Psychiatry 50:313–322
Penttilä J, Kajander J, Aalto S, Hirvonen J, Någren K, Ilonen T, Syvälahti E, Hia J et al (2004) Effects of fluoxetine on dopamine D2 receptors in the human brain—a positron emission tomography study with [11C]raclopride. Int J Neuropsychopharmacol 7:431–439
Rinne JO, Hia J, Ruotsalainen U, Sako E, Laihinen A, Någren K, Lehikoinen P, Oikonen V, Syvälahti E et al (1993) Decrease of human striatal dopamine D2 density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab 13:310–314
Schildkraut JJ (1965) The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry 122:509–522
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27
Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP (1997) Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol Med 127:1247–1256
Talvik M, Nordström AL, Okubo Y, Olsson H, Borg J, Halldin C, Farde L (2006) Dopamine D2 receptor binding in drug-naïve patients with schizophrenia examined with raclopride-C11 and positron emission tomography. Psychiatry Res 148(2–3):165–73
Tiihonen J, Kuoppamaki M, Någren K, Bergman J, Eronen E, Syvälahti E, Hia J et al (1996) Serotonergic modulation of striatal D2 dopamine receptor binding in humans measured with positron emission tomography. Psychopharmacology 126:277–280
Tzschentke TM (2001) Pharmacology and behavioural pharmacology of the mesocortical dopamine system. Prog Neurobiol 63:241–320
Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, Wilson AA (2006) High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry 59:389–394
Acknowledgments
This study was financially supported by Signe and Ane Gyllenberg Foundation, The Social Insurance Institution of Finland, and the Turku University Central Hospital (grant P3848). The staffs of Turku PET Centre and the MRI Unit of Turku University Central Hospital are acknowledged for skillful assistance in performing PET and MRI scanning.
Financial disclosures
Dr. Hirvonen has received lecture fees from AstraZeneca, Bristol-Myers Squibb, Janssen-Cilag, Lundbeck, and Novartis, congress travel grants from AstraZeneca and Lundbeck, and research funding from Orion Pharma and Lundbeck. Dr. Karlsson has received lecture fees from AstraZeneca, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Lundbeck, and Wyeth. Dr. Markkula has received lecture fees from Eli Lilly, GlaxoSmithKline, and Janssen-Cilag. Dr. Hietala has received lecture fees from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, and Lundbeck, congress travel grants from AstraZeneca, Bristol-Myers Squibb, and Eli Lilly, and has acted as a consultant for Orion Pharma. Drs. Kajander, Rasi-Hakala, Någren, and Salminen report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirvonen, J., Karlsson, H., Kajander, J. et al. Striatal dopamine D2 receptors in medication-naive patients with major depressive disorder as assessed with [11C]raclopride PET. Psychopharmacology 197, 581–590 (2008). https://doi.org/10.1007/s00213-008-1088-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1088-9