Abstract
Rationale
Ketamine induces effects resembling both positive and negative psychotic symptoms of schizophrenia. These are thought to arise through its action as an uncompetitive antagonist of the N-methyl-D-aspartate (NMDA) receptor.
Objectives
We used [123I]CNS-1261 to study ketamine binding to NMDA receptors in healthy human controls in vivo and its relationship to positive and negative psychotic symptom induction.
Materials and methods
Ten healthy controls underwent two single-photon emission tomography scans with [123I]CNS-1261. On each occasion, they received a bolus infusion of either ketamine or saline. The Brief Psychiatric Rating Scale (BPRS) was administered at the end of each scan. Predefined regions of interest were used to estimate change in volume of distribution of [123I]CNS-1261 following ketamine administration. Two normalised-to-cortex binding indices were also used in order to study effects of ketamine on NMDA receptor availability by region, after correction for global and nonspecific effects.
Results
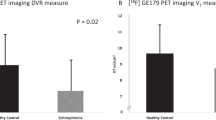
Ketamine-induced reduction in [123I]CNS-1261 volume of distribution in all regions showed the strongest correlation with BPRS negative subscale (p < 0.01). With the normalised-to-cortex measures, NMDA receptor binding in middle inferior frontal cortex showed a significant correlation with BPRS negative subscale (BI1 r = 0.88, BI2 r = 95.9, p < 0.001).
Conclusions
[123I]CNS-1261 binding was modulated by ketamine, a drug known to compete for the same site on the NMDA receptor in vitro. Ketamine may induce negative symptoms through direct inhibition of the NMDA receptor, and positive symptoms may arise through a different neurochemical pathway.


Similar content being viewed by others
References
Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H, Nagren K, Vilkman H, Gustafsson LL, Syvalahti E, Hietala> J (2005) Cortical glutamate–dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 182:375–383
Breier A, Adler CM, Weisenfeld N, Su TP, Elman I, Picken L, Malhotra AK, Pickar D (1998) Effects of NMDA antagonism on striatal dopamine release in healthy subjects: application of a novel PET approach. Synapse 29:142–147
Bressan RA, Erlandsson K, Mulligan RS, Gunn RN, Cunningham VJ, Owens J, Cullum ID, Ell PJ, Pilowsky LS (2004) A bolus/infusion paradigm for the novel NMDA receptor SPET tracer [123I]CNS 1261. Nucl Med Biol 31:155–164
Coyle JT, Tsai G, Goff D (2003) Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci 1003:318–327
Deutsch SI, Rosse RB, Schwartz BL, Mastropaolo J (2001) A revised excitotoxic hypothesis of schizophrenia: therapeutic implications. Clin Neuropharmacol 24:43–49
Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM (1997) Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol 333:99–104
Erlandsson K, Bressan RA, Mulligan RS, Gunn RN, Cunningham VJ, Owens J, Wyper D, Ell PJ, Pilowsky LS (2003) Kinetic modelling of [123I]CNS 1261—a potential SPET tracer for the NMDA receptor. Nucl Med Biol 30:441–454
Holcomb HH, Lahti AC, Medoff DR, Weiler M, Tamminga CA (2001) Sequential regional cerebral blood flow brain scans using PET with H2(15)O demonstrate ketamine actions in CNS dynamically. Neuropsychopharmacology 25:165–172
Javitt DC (2006) Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr Opin Psychiatry 19:151–157
Kapur S, Seeman P (2002) NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors—implications for models of schizophrenia. Mol Psychiatry 7:837–844
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr., Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Krystal JH, Perry EB Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D’Souza DC (2005) Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry 62:985–994
Logan J, Volkow ND, Fowler JS, Wang GJ, Dewey SL, MacGregor R, Schlyer D, Gatley SJ, Pappas N, King P et al (1994) Effects of blood flow on [11C]raclopride binding in the brain: model simulations and kinetic analysis of PET data. J Cereb Blood Flow Metab 14:995–1010
Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA (2003) Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117:697–706
Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A (1996) NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 14:301–307
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–1927
Olney JW, Farber NB (1995) Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 52:998–1007
Olney JW, Newcomer JW, Farber NB (1999) NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 33:523–533
Owens J, Tebbutt AA, McGregor AL, Kodama K, Magar SS, Perlman ME, Robins DJ, Durant GJ, McCulloch J (2000) Synthesis and binding characteristics of N-(1-naphthyl)-N′-(3-[(125)I]-iodophenyl)-N′-methylguanidine ([(125)I]-CNS 1261): a potential SPECT agent for imaging NMDA receptor activation. Nucl Med Biol 27:557–564
Pilowsky LS, Bressan RA, Stone JM, Erlandsson K, Mulligan RS, Krystal JH, Ell PJ (2006) First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry 11:118–119
Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM (2005) Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 162:394–396
Seeman P, Ko F, Tallerico T (2005) Dopamine receptor contribution to the action of PCP, LSD and ketamine psychotomimetics. Mol Psychiatry 10:877–883
Sharp FR, Tomitaka M, Bernaudin M, Tomitaka S (2001) Psychosis: pathological activation of limbic thalamocortical circuits by psychomimetics and schizophrenia? Trends Neurosci 24:330–334
Smith GS, Schloesser R, Brodie JD, Dewey SL, Logan J, Vitkun SA, Simkowitz P, Hurley A, Cooper T, Volkow ND, Cancro R (1998) Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology 18:18–25
Stone JM, Pilowsky LS (2006) Psychopathological consequences of ketamine. Br J Psychiatry 189:565–566
Stone JM, Erlandsson K, Arstad E, Bressan RA, Squassante L, Teneggi V, Ell PJ, Pilowsky LS (2006) Ketamine displaces the novel NMDA receptor SPET probe [(123)I]CNS-1261 in humans in vivo. Nucl Med Biol 33:239–243
Szulc A, Galinska B, Tarasow E, Dzienis W, Kubas B, Konarzewska B, Walecki J, Alathiaki AS, Czernikiewicz A (2005) The effect of risperidone on metabolite measures in the frontal lobe, temporal lobe, and thalamus in schizophrenic patients. A proton magnetic resonance spectroscopy (1H MRS). Pharmacopsychiatry 38:214–219
Takeuchi R, Yonekura Y, Matsuda H, Konishi J (2002) Usefulness of a three-dimensional stereotaxic ROI template on anatomically standardised 99mTc-ECD SPET. Eur J Nucl Med Mol Imaging 29:331–341
Tuominen HJ, Tiihonen J, Wahlbeck K (2005) Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr Res 72:225–234
Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA (2000) Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res 97:129–135
Vollenweider FX, Geyer MA (2001) A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull 56:495–507
Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL (2000) Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res 34:35–43
Acknowledgements
LSP, EA, KE were supported by a UK Medical Research Council Senior Clinical Research Fellowship to LSP. JS and RAB were supported by a research grant from GlaxoSmithKline. JS is currently a Medical Research Council Clinical Research Training Fellow. JHK was funded by US National Institute on Alcohol Abuse and Alcoholism (KO5 AA 14906–01), US Department of Veterans Affairs Schizophrenia Biological Research Center and Alcohol Research Center. All experiments were performed with full ethical approval, in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stone, J.M., Erlandsson, K., Arstad, E. et al. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy—a [123I]CNS-1261 SPET study. Psychopharmacology 197, 401–408 (2008). https://doi.org/10.1007/s00213-007-1047-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-1047-x