Abstract
Rationale
Acute pharmacological studies implicate μ-opioid receptors (MORs) in the parabrachial nucleus (PBN) of the brainstem in modulating eating. The long-term effects of preventing the cellular function of parabrachial MORs on food consumption remain to be elucidated.
Objectives
To determine whether (1) chronic inhibition of MOR-mediated G-protein coupling in the PBN of rats would persistently reduce eating and (2) food properties dictate the effects of MOR blockade.
Materials and methods
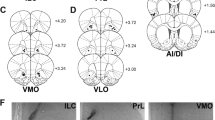
We microinfused the irreversible MOR antagonist, β-funaltrexamine (β-FNA) into the lateral PBN and measured the intake of standard and calorically dense palatable chow for 1 week. First, rats were given standard chow for 20 h daily and a calorically dense palatable chow for 4 h during the day. We infused the agonist, [d-Ala2, N-Me-Phe4, Glycinol5]-Enkephalin (DAMGO), 1 week after β-FNA to probe the acute effects of exogenous stimulation of MORs on palatable food intake. [35S]GTPγS autoradiography quantified regional loss of MOR cellular function. Next, we measured the actions of β-FNA on food intake in rats given only standard or palatable chow for 1 week.
Results
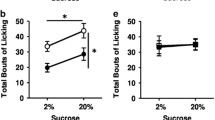
One infusion of β-FNA persistently decreased consumption of standard but not palatable chow, regardless of feeding regimen. β-FNA also blocked DAMGO-stimulated palatable chow intake, prevented DAMGO-stimulated G-protein coupling in the central and external lateral subnuclei of the PBN, and decreased coupling in the medial PBN. β-FNA did not affect κ-opioid receptors.
Conclusions
MORs in the lateral PBN serve a physiological role in stimulating consumption of standard food. Properties of the diet, such as high palatability or caloric density, may override the influence of inhibiting MOR function.








Similar content being viewed by others
References
Arjune D, Standifer KM, Pasternak GW, Bodnar RJ (1990) Reduction by central beta-funaltrexamine of food intake in rats under freely-feeding, deprivation and glucoprivic conditions. Brain Res 535:101–109
Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Loh HH, Elde R, Wessendorf MW (1995) delta-Opioid receptor immunoreactivity: distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J Neurosci 15:1215–1235
Baird JP, Travers SP, Travers JB (2001) Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281:R1581–R1593
Bakshi VP, Kelley AE (1993) Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 111:207–214
Berthoud HR (2004) Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite 43:315–317
Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML (1995) General, m and k opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res 700:205–212
Bodnar RJ, Lamonte N, Israel Y, Kandov Y, Ackerman TF, Khaimova E (2005) Reciprocal opioid-opioid interactions between the ventral tegmental area and nucleus accumbens regions in mediating mu agonist-induced feeding in rats. Peptides 26:621–629
Calcagnetti D, Reid L (1983) Morphine and acceptability of putative reinforcers. Pharmacol Biochem Behav 18:567–569
Calingasan NY, Ritter S (1993) Lateral parabrachial subnucleus lesions abolish feeding induced by mercaptoacetate but not by 2-deoxy-D-glucose. Am J Physiol 265:R1168–R1178
Cechetto DF, Saper CB (1987) Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol 262:27–45
Cole JL, Leventhal L, Pasternak GW, Bowen WD, Bodnar RJ (1995) Reductions in body weight following chronic central opioid receptor subtype antagonists during development of dietary obesity in rats. Brain Res 678:168–176
Corwin RL, Hajnal A (2005) Too much of a good thing: neurobiology of non-homeostatic eating and drug abuse. Physiol Behav 86:5–8
Evans KR, Vaccarino FJ (1990) Amphetamine- and morphine-induced feeding: evidence for involvement of reward mechanisms. Neurosci Biobehav Rev 14:9–22
Fratucci De Gobbi JL, De Luca LA Jr, Johnson AK, Menani JV (2001) Interaction of serotonin and cholecystokinin in the lateral parabrachial nucleus to control sodium intake. Am J Physiol Regul Integr Comp Physiol 280:R1301–R1307
Fulwiler CE, Saper CB (1984) Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 319:229–259
Gosnell BA, Lipton JM (1986) Opioid peptide effects on feeding in rabbits. Peptides 7:745–747
Gosnell BA, Majchrzak M (1990) Effects of a selective mu opioid receptor agonist and naloxone on the intake of sodium chloride solutions. Psychopharmacology (Berl) 100:66–71
Guide for the care and use of laboratory animals (2003) National Institutes of Health, Maryland, National Research Council/Public Health Service
Halsell CB, Frank ME (1991) Mapping study of the parabrachial taste-responsive area for the anterior tongue in the golden hamster. J Comp Neurol 306:708–722
Herbert H, Moga MM, Saper CB (1990) Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293:540–580
Hermann GE, Rogers RC (1985) Convergence of vagal and gustatory afferent input within the parabrachial nucleus of the rat. J Auton Nerv Syst 13:1–17
Karimnamazi H, Travers JB (1998) Differential projections from gustatory responsive regions of the parabrachial nucleus to the medulla and forebrain. Brain Res 813:283–302
Karimnamazi H, Travers SP, Travers JB (2002) Oral and gastric input to the parabrachial nucleus of the rat. Brain Res 957:193–206
Kirkham TC, Cooper SJ (1988a) Attenuation of sham feeding by naloxone is stereospecific: evidence for opioid mediation of orosensory reward. Physiol Behav 43:845–847
Kirkham TC, Cooper SJ (1988b) Naloxone attenuation of sham feeding is modified by manipulation of sucrose concentration. Physiol Behav 44:491–494
Koch JE, Glass MJ, Cooper ML, Bodnar RJ (1995) Alterations in deprivation, glucoprivic and sucrose intake following general, mu and kappa opioid antagonists in the hypothalamic paraventricular nucleus of rats. Neuroscience 66:951–957
Larson DL, Hua M, Takemori AE, Portoghese PS (1993) Possible contribution of a glutathione conjugate to the long-duration action of beta-funaltrexamine. J Med Chem 36:3669–3673
Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ (1995) Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol Regul 268:R248–R252
Liu-Chen LY, Li SX, Lewis ME (1991) Autoradiographic study of irreversible binding of [3H]beta-funaltrexamine to opioid receptors in the rat forebrain: comparison with mu and delta receptor distribution. Brain Res 544:235–242
Majeed NH, Przewlocka B, Wedzony K, Przewlocki R (1986) Stimulation of food intake following opioid microinjection into the nucleus accumbens septi in rats. Peptides 7:711–716
Martin G, Nie Z, Siggins GR (1997) mu-Opioid receptors modulate NMDA receptor-mediated responses in nucleus accumbens neurons. J Neurosci 17:11–22
Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB (1990a) Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol 295:624–661
Moga MM, Saper CB, Gray TS (1990b) Neuropeptide organization of the hypothalamic projection to the parabrachial nucleus in the rat. J Comp Neurol 295:662–682
Mucha RF, Iversen SD (1986) Increased food-intake after opioid microinjections into the nucleus accumbens and ventral tegmental area of rat. Brain Res 397:214–224
Nicklous DM, Simansky KJ (2003) Neuropeptide FF exerts pro- and anti-opioid actions in the parabrachial nucleus to modulate food intake. Am J Physiol Regul Integr Comp Physiol 285:R1046–R1054
Norgren R, Pfaffman C (1975) The pontine taste area in the rat. Brain Res 91:99–117
Ogawa H, Hayama T, Ito S (1987) Response properties of the parabrachio-thalamic taste and mechanoreceptive neurons in rats. Exp Brain Res 68:449–457
Portoghese PS, Larson DL, Sayre LM, Fries DS, Takemori AE (1980) A novel opioid receptor site directed alkylating agent with irreversible narcotic antagonistic and reversible agonistic activities. J Med Chem 23:233–234
Saper CB (1995) Central autonomic system, 2nd edn. Academic New York
Sim LJ, Selley DE, Childers SR (1995) In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[gamma-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA 92:7242–7246
Simansky KJ (2005) NIH symposium series: ingestive mechanisms in obesity, substance abuse and mental disorders. Physiol Behav 86:1–4
Stanley BG, Lanthier D, Leibowitz SH (1989) Multiple brain sites sensitive to feeding stimulation by opioid agonist: a cannula-mapping study. Pharmacol Biochem Behav 31:825–832
Travers SP, Smith DV (1984) Responsiveness of neurons in the hamster parabrachial nuclei to taste mixtures. J Gen Physiol 84:221–250
Trifunovic R, Reilly S (2002) Medial versus lateral parabrachial nucleus lesions in the rat: effects on mercaptoacetate-induced feeding and conditioned taste aversion. Brain Res Bull 58:107–113
Ward SJ, Takemori AE (1983) Relative involvement of receptor subtypes in opioid-induced inhibition of gastrointestinal transit in mice. J Pharmacol Exp Ther 224:359–363
Ward SJ, Portoghese PS, Takemori AE (1982) Pharmacological characterization in vivo of the novel opiate, beta-funaltrexamine. J Pharmacol Exp Ther 220:494–498
Ward HG, Nicklous DM, Aloyo VJ, Simansky KJ (2006) Mu-opioid receptor cellular function in the nucleus accumbens is essential for hedonically driven eating. Eur J Neurosci 23:1605–1613
Will MJ, Franzblau EB, Kelley AE (2003) Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci 23:2882–2888
Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ (2003) An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 285:R1055–R1065
Yamamoto T, Sawa K (2000a) c-Fos-like immunoreactivity in the brainstem following gastric loads of various chemical solutions in rats. Brain Res 866:135–143
Yamamoto T, Sawa K (2000b) Comparison of c-fos-like immunoreactivity in the brainstem following intraoral and intragastric infusions of chemical solutions in rats. Brain Res 866:144–151
Zhang M, Kelley AE (1997) Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 132:350–360
Zhang M, Kelley AE (2002) Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 159:415–423
Zhu J, Luo LY, Li JG, Chen C, Liu-Chen LY (1997) Activation of the cloned human kappa opioid receptor by agonists enhances [35S]GTPgammaS binding to membranes: determination of potencies and efficacies of ligands. J Pharmacol Exp Ther 282:676–684
Acknowledgements
The authors thank Dr. Vincent Aloyo for his helpful comments about the manuscript and Alicia Spor and Adam Phillips for their technical assistance. This work was supported by USPHS Grant DK067648 from the National Institute of Diabetes and Digestive and Kidney Diseases to KJS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ward, H.G., Simansky, K.J. Chronic prevention of μ-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology 187, 435–446 (2006). https://doi.org/10.1007/s00213-006-0463-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0463-7