Abstract
Rationale
Dopamine β-hydroxylase (DBH) converts dopamine (DA) to norepinephrine (NE), thus playing a critical role in catecholamine metabolism.
Objectives/Methods
We examined the effects of Dbh gene dosage and the DBH inhibitor disulfiram in mice with zero, one, or two null Dbh alleles (+/+, +/−, and −/− mice).
Results
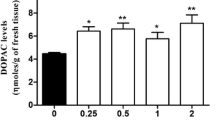
DBH protein levels in adrenal and prefrontal cortex (PFC) and adrenal DBH activity were proportional to number of wild-type alleles. Adrenal DA was slightly increased in +/− mice and markedly increased (80-fold) in −/− mice compared to wild-type animals. While adrenal NE and epinephrine (EPI) were undetectable in −/− mice, adrenal concentrations of NE and EPI were similar in +/+ and +/− mice, suggesting that the increase in DA maintains the normal rate of β-hydroxylation in Dbh +/− mice. Disulfiram had little effect on adrenal catecholamine levels, regardless of genotype or dose. NE was absent in the PFC of −/− mice, but only slightly reduced in +/− animals compared to wild-type animals. PFC DA was increased twofold in +/− mice and fivefold in −/− mice, and the NE to DA ratio was reduced (∼35%) in +/− mice, compared to wild-type mice. Disulfiram significantly decreased PFC NE and increased DA in +/+ and +/− animals, with the disulfiram and genotype effects on the PFC NE to DA ratio apparently additive.
Conclusions
The data reveal potentially important and apparently additive effects of Dbh genotype and disulfiram administration on PFC catecholamine metabolism. These effects may have implications for genetic control of DBH activity in humans and for understanding therapeutic effects of disulfiram.





Similar content being viewed by others
References
Andersson K, Fuxe K, Agnati LF (1985) Determinations of catecholamine half-lives and turnover rates in discrete catecholamine nerve terminal systems of the hypothalamus, the preoptic region and the forebrain by quantitative histofluorimetry. Acta Physiol Scand 123:411–426
Benowitz NL (1993) Clinical pharmacology and toxicology of cocaine. Pharmacol Toxicol 72:3–12
Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ (1998) Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction 93:713–727
Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ (2004) Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry 61:264–272
Cubells JF, Zabetian CP (2004) Human genetics of plasma dopamine beta-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology (Berl.) 174:463–476
Cubells JF, van Kammen DP, Kelley ME, Anderson GM, O'Connor DT, Price LH, Malison R, Rao PA, Kobayashi K, Nagatsu T, Gelernter J (1998) Dopamine beta-hydroxylase: two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Hum Genet 102:533–540
Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, Gelernter J (2000) A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Mol Psychiatry 5:56–63
Ewing JA, Mueller RA, Rouse BA, Silver D (1977) Low levels of dopamine beta-hydroxylase and psychosis. Am J Psychiatry 134:927–928
Ewing JA, Rouse BA, Mueller RA, Silver D (1978) Can dopamine beta-hydroxylase levels predict adverse reactions to disulfiram? Alcohol Clin Exp Res 2:93–94
George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS (2000) Disulfiram vs. placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol Psychiatry 47:1080–1086
Goldstein M (1966) Inhibition of noradrenaline biosynthesis at the dopamine-beta-hydroxylation stage. Pharmacol Rev 18:77–82
Goldstein M, Anagnoste B, Lauber E, McKereghan MR (1964) Inhibition of dopamine β-hydroxylase by disulfiram. Life Sci 3:763–767
Haile CN, During MJ, Jatlow PI, Kosten TR, Kosten TA (2003) Disulfiram facilitates the development and expression of locomotor sensitization to cocaine in rats. Biol Psychiatry 54:915–921
Hameedi FA, Rosen MI, McCance-Katz EF, McMahon TJ, Price LH, Jatlow PI, Woods SW, Kosten TR (1995) Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biol Psychiatry 37:560–563
Heath RG, Nesselhof W, Bishop MR, Byers M (1965) Behavioral and metabolic changes associated with administration of tetraethylthiuram disulfide (Antabuse). Dis Nerv Syst 26:99–105
Hoeldtke RD, Stetson PL (1980) An in vivo tritium release assay of human dopamine beta-hydroxylase. J Clin Endocrinol Metab 51:810–815
Johansson B (1992) A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr Scand 369(Suppl):15–26
Jonsson EG, Bah J, Melke J, Abou Jamra R, Schumacher J, Westberg L, Ivo R, Cichon S, Propping P, Nothen MM, Eriksson E, Sedvall GC (2004) Monoamine related functional gene variants and relationships to monoamine metabolite concentrations in CSF of healthy volunteers. BMC Psychiatry 4:4
Karamanakos PN, Pappas P, Stephanou P, Marselos M (2001) Differentiation of disulfiram effects on central catecholamines and hepatic ethanol metabolism. Pharmacol Toxicol 88:106–110
Koster G (1986) Time course of the metabolite patterns of intraventricularly injected [3H]noradrenaline in rat brain regions. J Neurochem 47:1132–1140
Major LF, Lerner P, Ballenger JC, Brown GL, Goodwin FK, Lovenberg W (1979) Dopamine-beta-hydroxylase in the cerebrospinal fluid: relationship to disulfiram-induced psychosis. Biol Psychiatry 14:337–344
McCance-Katz EF, Kosten TR, Jatlow P (1998a) Disulfiram effects on acute cocaine administration. Drug Alcohol Depend 52:27–39
McCance-Katz EF, Kosten TR, Jatlow P (1998b) Chronic disulfiram treatment effects on intranasal cocaine administration: initial results. Biol Psychiatry 43:540–543
Musacchio JM, Goldstein M, Anagnoste B, Poch G, Kopin IJ (1966) Inhibition of dopamine-beta-hydroxylase by disulfiram in vivo. J Pharmacol Exp Ther 152:56–61
Nagatsu T, Udenfriend S (1972) Photometric assay of dopamine-β-hydroxylase activity in human blood. Clin Chem 18:980–983
Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ (2000) Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction 95:219–228
Robertson D, Haile V, Perry SE, Robertson RM, Phillips JA III, Biaggioni I (1991) Dopamine beta-hydroxylase deficiency. A genetic disorder of cardiovascular regulation. Hypertension 18:1–8
Scatton B, Dennis T, Curet O (1984) Increase in dopamine and DOPAC levels in noradrenergic terminals after electrical stimulation of the ascending noradrenergic pathways. Brain Res 298:193–196
Stewart DJ, Inaba T, Lucassen M, Kalow W (1979) Cocaine metabolism: cocaine and norcocaine hydrolysis by liver and serum esterases. Clin Pharmacol Ther 25:464–468
Tassin JP (1998) Norepinephrine-dopamine interactions in the prefrontal cortex and the ventral tegmental area: relevance to mental diseases. Adv Pharmacol 42:712–716
Tassin JP, Trovero F, Herve D, Blanc G, Glowinski J (1992) Biochemical and behavioural consequences of interactions between dopaminergic and noradrenergic systems in rat prefrontal cortex. Neurochem Int 20(Suppl):225S–230S
Thomas SA, Matsumoto AM, Palmiter RD (1995) Noradrenaline is essential for mouse fetal development. Nature 374:643–646
Thomas SA, Marck BT, Palmiter RD, Matsumoto AM (1998) Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem 70:2468–2476
Trovero F, Marin P, Tassin JP, Premont J, Glowinski J (1994) Accelerated resensitization of the D1 dopamine receptor-mediated response in cultured cortical and striatal neurons from the rat: respective role of alpha 1-adrenergic and N-methyl-D-aspartate receptors. J Neurosci 14:6280–6288
Udenfriend S, Wyngaarden JB (1956) Precursors of adrenal epinephrine and norepinephrine in vivo. Biochim Biophys Acta 20:48–52
Udenfriend S, Cooper JR, Clark CT, Baer JE (1953) Rate of turnover of epinephrine in the adrenal medulla. Science 117:663–665
Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD (2002) Mice with chronic noradrenaline deficiency resemble amphetamine-sensitized animals. Proc Natl Acad Sci U S A 99:13873–13977
Weinshilboum RM (1979) Serum dopamine β-hydroxylase. Pharmacol Rev 30:133–166
Zabetian CP et al (2001) A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet 68:515–522
Acknowledgements
We thank Porche' Kirkland for technical assistance, Dr. Betty Eipper and Dr. Steven Ebert for providing DBH antibody, and Sumitomo Pharmaceuticals (Osaka, Japan) for their generous donation of the DOPS required to breed the Dbh −/− mice. Z.R.D. was funded by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
B.N. Bourdélat-Parks and G.M. Anderson contributed equally to this work
Rights and permissions
About this article
Cite this article
Bourdélat-Parks, B.N., Anderson, G.M., Donaldson, Z.R. et al. Effects of dopamine β-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology 183, 72–80 (2005). https://doi.org/10.1007/s00213-005-0139-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0139-8