Abstract
Rationale
Lesions of the orbital prefrontal cortex (OPFC) can cause pathologically impulsive behaviour in humans. Inter-temporal choice behaviour (choice between reinforcers differing in size and delay) has been proposed as a model of “impulsive choice” in animals. We recently found that destruction of the OPFC disrupted inter-temporal choice in rats. It is not known whether the dopaminergic projection to the OPFC contributes to the regulation of inter-temporal choice.
Objective
A quantitative method was used to compare inter-temporal choice in rats whose OPFC had been depleted of dopamine with that of sham-lesioned control rats.
Methods
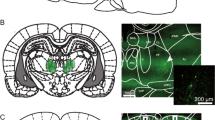
Under halothane anaesthesia, rats received injections of 6-hydroxydopamine into the OPFC (2 μg μl−1, 0.5 μl, two injections in each hemisphere), or sham lesions (injections of the vehicle). They were trained to press two levers (A and B) for sucrose reinforcement (0.6 M) in discrete-trials schedules. In free-choice trials, a press on A resulted in delivery of 50 μl of the sucrose solution after a delay d A; a press on B resulted in delivery of 100 μl of the same solution after a delay d B. d B was increased progressively across successive blocks of six trials in each session, while d A was manipulated systematically across phases of the experiment. The indifference delay, d B(50) (value of d B corresponding to 50% choice of B) was estimated for each rat in each phase. Linear functions of d B(50) versus d A were derived, and the parameters of the function compared between the groups. Concentrations of monoamines in the OPFC were determined by high-performance liquid chromatography at the end of the experiment.
Results
In both groups, d B(50) increased linearly with d A (r 2>0.9 in each case). The slope of the function was significantly steeper in the lesioned group than the sham-lesioned group, whereas the intercept did not differ significantly between the groups. When delays of 4 or 8 s were imposed on the smaller reinforcer, the lesioned rats showed greater tolerance of delay to the larger reinforcer (i.e. they exhibited longer values of d B(50)) than the sham-lesioned rats. Dopamine, noradrenaline and 5-hydroxytryptamine levels in the OPFC of the lesioned group were 20, 75 and 98% of those of the sham-lesioned group.
Conclusions
The results indicate that dopaminergic afferents to the OPFC contribute to the regulation of inter-temporal choice behaviour due to their role in determining organisms’ sensitivity both to reinforcer size and to delay of reinforcement.




Similar content being viewed by others
References
Ainslie GW (1975) Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull 82:463–492
Cardinal RN, Robbins TW, Everitt BJ (2000) The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delay of reinforcement in rats. Psychopharmacology 152:362–375
Cardinal RN, Pennicott DR, Lakmali Sugathapala C, Robbins TW, Everitt BJ (2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292:2499–2501
Dietrich A, Allen J (1998) Functional dissociation of the prefrontal cortex and hippocampus in timing behaviour. Behav Neurosci 112:1043–1047
Dietrich A, Frederick DL, Allen J (1997) The effect of total and subtotal prefrontal cortex lesions on the timing ability of the rat. Psychobiology 25:191–201
Evenden JL (1999a) Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol 13:180–192
Evenden JL (1999b) Varieties of impulsivity. Psychopharmacology 146:348–361
Evenden JL, Ryan CN (1996) The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 128:161–170
Evenden JL, Ryan CN (1999) The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and sedative serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology 146:413–421
Gallagher M, McMahon RW, Schoenbaum G (1999) Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci 19:6610–6614
Gibbon J, Church RM, Fairhurst S, Kacelnik A (1988) Scalar expectancy and choice between delayed rewards. Psychol Rev 95:102–114
Grace RC (1994) A contextual model of concurrent chains choice. J Exp Anal Behav 61:113–129
Groenewegen HJ, Uylings HBM (2000) The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res 85:31–62
Heffner TG, Hartman JA, Seiden LS (1988) A method for regional dissection of the rat brain. Pharmacol Biochem Behav 13:453–456
Herrnstein RJ (1981) Self-control as response strength. In: Bradshaw CM, Szabadi E, Lowe CF (eds) Quantification of steady-state operant behaviour. Elsevier, Amsterdam, pp 3–20
Ho M-Y, Bradshaw CM, Szabadi E (1997) Choice between delayed reinforcers: interaction between delay and deprivation level. Q J Exp Psychol 50B:193–202
Ho M-Y, Al-Zahrani SSA, Al-Ruwaitea ASA, Bradshaw CM, Szabadi E (1998) 5-Hydroxytryptamine and impulse control: prospects for a behavioural analysis. J Psychopharmacol 12:68–78
Ho M-Y, Mobini S, Chiang T-J, Bradshaw CM, Szabadi E (1999) Theory and method in the quantitative analysis of impulsive choice behaviour: implications for psychopharmacology. Psychopharmacology 146:362–372
Iversen SD, Mishkin M (1970) Perseverative interference in monkey following selective lesions of the inferior prefrontal convexity. Exp Brain Res 11:376–386
Jones B, Mishkin M (1972) Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurol 36:362–377
Kacelnik A (1997) Normative and descriptive models of decision making: time discounting and risk sensitivity. Ciba Foundation Symposium, 208: Characterizing human psychological adaptations. Wiley, Chichester
Kheramin S, Body S, Mobini S, Ho M-Y, Velazquez Martinez DN, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM (2002) Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: a quantitative analysis. Psychopharmacology 165:9–17
Kheramin S, Body S, Ho M-Y, Velazquez Martinez DN, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM (2003) Role of the orbital prefrontal cortex in choice between delayed and uncertain reinforcers: a quantitative analysis. Behav Proc 64:239–250
Kolb B (1984) Functions of the frontal cortex of the rat: a comparative review. Brain Res Rev 8:65–98
Kolb B (1990) Animal models for human PFC-related disorders. In: Uylings CG, Van Eden CG, De Bruin JPC, Corner MA, Feenstra MGP (eds) Progress in brain research, vol 85. Elsevier, Amsterdam, pp 501–520
Lishman WA (1998) Organic psychiatry, 3rd edn. Blackwell Science, Oxford
Logue AW (1988) Research on self-control: an integrated framework. Behav Brain Sci 11:665–709
Mazur JE (1987) An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin HC (eds) Quantitative analyses of behavior, vol 5. The effect of delay and intervening events on reinforcement value. Erlbaum, Hillsdale, N.J., pp 55–73
Mazur JE (1995) Conditioned reinforcement and choice with delayed and uncertain primary reinforcers. J Exp Anal Behav 63:139–150
Mazur JE (1997) Choice, delay, probability, and conditioned reinforcement. Anim Learn Behav 25:131–147
Mischel W (1983) Delay of gratification as a process and a person variable in development. In: Magnussen D, Allen VL (eds) Human development. Academic Press, New York
Mobini S, Chiang T-J, Al-Ruwaitea ASA, Ho M-Y, Bradshaw CM, Szabadi E (2000a) Effects of central 5-hydroxytryptamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology 149:313–318
Mobini S, Body S, Ho M-Y, Bradshaw CM, Szabadi E (2000b) Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology 160:290–298
Mobini S, Chiang T-J, Ho M-Y, Bradshaw CM, Szabadi E (2000c) Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology 152:390–397
Monterosso J, Ainslie GW (1999) Beyond discounting: possible experimental models of impulse control. Psychopharmacology 146:339–347
Oades RD, Halliday GM (1987) Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res Rev 12:117–165
Otto T, Eichenbaum H (1992) Complementary roles of the orbital prefrontal cortex and perirhinal–entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behav Neurosci 106:762–775
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, New York
Rachlin H (1974) Self-control. Behaviorism 2:94–107
Rachlin H (1995) Self-control: beyond commitment. Behav Brain Sci 18:109–159
Richards JB, Mitchell SH, de Wit H, Seiden LS (1997) Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav 67:353–366
Richards JB, Sabol KE, de Wit H (1999) Effects of methamphetamine and the adjusting amount procedure: a model of impulsive beavior in rats. Psychopharmacology 146:432–439
Rolls ET (1999) The brain and emotion. Oxford University Press, Oxford
Rolls ET (2000) The orbitofrontal cortex and reward. Cereb Cortex 10:284–294
Rushworth MFS, Nixon PD, Eacott MJ, Passingham RE (1997) Ventral prefrontal cortex is not essential for working memory. J Neurosci 17:4829–4838
Schoenbaum G, Nugent SL, Saddoris MP, Setlow B (2002) Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discrimination. Neuroreport 13:885–890
Schultz W (2000) Multiple reward signals in the brain. Nature Neurosci 1:199–207
Schultz W (2002) Getting formal with dopamine and reward. Neuron 36:241–263
Tzschentke TM (2001) Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol 63:241–320
Wade TR, de Wit H, Richards JB (2000) Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology 150:90–101
Winer BJ (1991) Statistical principles in experimental design, 3rd edn. McGraw-Hill, New York
de Wit H, Engagasser JL, Richards JB (2002) Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 27:813–825
Wogar MA, Bradshaw CM, Szabadi E (1992) Impaired acquisition of temporal differentiation performance following lesions of the ascending 5-hydroxytryptaminergic pathways. Psychopharmacology 111:373–378
Zavitsanou K, Cranney J, Richardson R (1999) Dopamine antagonists in the orbital prefrontal cortex reduce prepulse inhibition of the acoustic startle reflex in the rat. Pharmacol Biochem Behav 63:55–61
Acknowledgements
This work was supported by a grant from the Wellcome Trust to C.M.B. and E.S. (University of Nottingham) and J.F.W.D. and I.M.A. (University of Manchester). D.N.V.-M. was supported by grants from CONACYT (#25090-H) and Universidad Nacional Autónoma de México DGAPA (#229981). We are grateful to Mrs. Victoria Bak and Mr. R.W. Langley for skilled technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kheramin, S., Body, S., Ho, MY. et al. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology 175, 206–214 (2004). https://doi.org/10.1007/s00213-004-1813-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-004-1813-y