Abstract
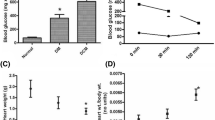
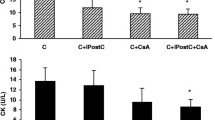
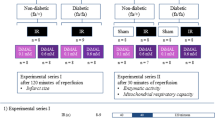
Our previous study demonstrated that hydrogen sulfide post-conditioning (HPOC) renders cardioprotection against ischemia-reperfusion (I/R) injury in normal rat by preserving mitochondria. But its efficacy in ameliorating I/R in the diabetic heart with (DCM) or without cardiomyopathy (DM) is unclear and is the focus of the present study. Normal (N), diabetes mellitus (streptozotocin, 35 mg/kg; normal diet), and DCM (streptozotocin, 35 mg/kg; high-fat diet) rats were subjected to I/R (30 min global ischemia followed by 60 min reperfusion) in presence and absence of HPOC using ex vivo Langendorff perfusion system. At the end of heart perfusion, subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM) fractions from the tissue were isolated and measured for the ATP production, electron transport chain (ETC) enzyme activity, and membrane potential. The prominent I/R-associated injury in DCM rat was not subsequently attenuated by HPOC protocol unlike in the normal or diabetic rat heart (latter rat heart showed moderate protection) (HPOC recovery on infarct size: N 75% vs. DM 63% vs. DCM 48%). The baseline ATP content and subsequent ATP-producing capacity in DCM rat heart were low as compared with those in normal or DM rat heart, especially in SSM. HPOC protocol reversed the I/R-induced low mitochondrial ATP content and low ATP-producing capacity (both in non-energized and energized with glutamate/malate) significantly in normal and DM hearts, but not in DCM heart. Moreover in DCM, decreased activity of mitochondrial electron chain enzymes (complexes I, II, III, and IV) in SSM (26%, 88%, 57%, and 17%) and IFM (76%, 89%, 60%, and 13%) from sham control was maintained even after the conditioning of heart with hydrogen sulfide donor. Results altogether suggest that significantly higher levels of perturbing mitochondria in DCM rat heart underline the deteriorated cardiac recovery by HPOC.





Similar content being viewed by others
References
Algeria JR, Miller TD, Gibbons RJ, Yi QL, Yusuf S (2007) Collaborative Organization of RheothRx Evaluation (CORE) Trial Investigators infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am Heart J 154:743–750
Ansari SB, Kurian GA (2016) Hydrogen sulfide modulates sub-cellular susceptibility to oxidative stress induced by myocardial ischemic reperfusion injury. Chem Biol Interact 252:28–35
Banu SA, Ravindran S, Kurian GA (2016) Hydrogen sulfide post-conditioning preserves interfibrillar mitochondria of rat heart during ischemia reperfusion injury. Cell Stress Chaperones 21(4):571–582
Baracca A, Sgarbi G, Solaini G, Lenaz G (2003) Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochim Biophys Acta Bioenerg 1606(1–3):137–146
Bibli SI, Andreadou I, Chatzianastasiou A, Tzimas C, Sanoudou D, Kranias E, Brouckaert P, Coletta C, Szabo C, Kremastinos DT, Iliodromitis EK (2015) Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc Res 106:432–442
Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ (2009) Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105:365–374
Chatzianastasiou A, Bibli SI, Andreadou I, Efentakis P, Kaludercic N, Wood ME, Whiteman M, Di Lisa F, Daiber A, Manolopoulos VG, Szabó C (2016) Cardioprotection by H2S donors: nitric oxide-dependent and -independent mechanisms. J Pharmacol Exp Ther 106:432–442
Chinopoulos C, Starkov AA, Fiskum G (2003) Cyclosporin A-insensitive permeability transition in brain mitochondria: inhibition by 2-aminoethoxydiphenyl borate. J Biol Chem 278:27382–27389
Croston TL, Thapa D, Holden AA, Tveter KJ, Lewis SE, Shepherd DL, Nichols CE, Long DM, Olfert IM, Jagannathan R, Hollander JM (2014) Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart. Am J Physiol Heart Circ Physiol 307(1):H54–H65
Dabkowski ER, Baseler WA, Williamson CL, Powell M, Ra-zunguzwa TT, Frisbee JC, Hollander JM (2010) Mito-chondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol 299:H529–H540
Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A 97:12222–12226
Duncan JG (2011) Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta Mol Cell Res 1813(7):1351–1359
Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H (2007) Hydrogen sulfide attenuates myocardial ischemia–reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A 104:15560–15565
Frazier AE, Thorburn DR (2012) Biochemical analyses of the electron transport chain complexes by spectrophotometry. Methods Mol Biol 837:49–62
Galderisi M, Anderson KM, Wilson PW, Levy D (1991) Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol 68:85–89
Hine C, Kim HJ, Zhu Y, Harputlugil E, Longchamp A, Matos MS, Ramadoss P, Bauerle K, Brace L, Asara JM, Ozaki CK (2017) Hypothalamic-pituitary axis regulates hydrogen sulfide production. Cell Metabol 25(6):1320–1333
Kurian GA, Berenshtein E, Kakhlon O, Chevion M (2012) Energy status determines the distinct biochemical and physiological behavior of interfibrillar and sub-sarcolemmal mitochondria. Biochem Biophys Res Commun 428(3):376–382
Lambert JP, Nicholson CK, Amin H, Amin S, Calvert JW (2014) Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med Gas Res 4:20–31
Mahalakshmi A, Kurian GA (2018) Evaluating the impact of diabetes and diabetic cardiomyopathy rat heart on the outcome of ischemia-reperfusion associated oxidative stress. Free Radic Biol Med 118:35–43
Marso SP, Miller T, Rutherford BD, Gibbons RJ, Qureshi M, Kalynych A, Turco M, Schultheiss HP, Mehran R, Krucoff MW, Lansky AJ (2007) Comparison of myocardial reperfusion in patients undergoing percutaneous coronary intervention in ST-segment elevation acute myocardial infarction with versus without diabetes mellitus (from the EMERALD trial). Am J Cardiol 100:206–210
Miki T, Yuda S, Kouzu H, Miura T (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18(2):149–166
Murphy E (2004) Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res 94(1):7–16
Palmer JW, Tandler B, Hoppel CL (1977) Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252(23):8731–8739
Peake BF, Nicholson CK, Lambert JP, Hood RL, Amin H, Amin S, Calvert JW (2013) Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am J Physiol Heart Circ Physiol 304(9):H1215–H1224
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30:595–602
Si YF, Wang J, Guan J, Zhou L, Sheng Y, Zhao J (2013) Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br J Pharmacol 169(3):619–631
Skulachev VP (2001) Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci 26:23–29
van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P (2011) Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res 92(1):10–18
Voulgari C, Papadogiannis D, Tentolouris N (2010) Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc Health Risk Manag 6:883
Ye P, Gu Y, Zhu YR, Chao YL, Kong XQ, Luo J, Ren XM, Zuo GF, Zhang DM, Chen SL (2018) Exogenous hydrogen sulfide attenuates the development of diabetic cardiomyopathy via the FoxO1 pathway. J Cell Physiol 233(12):9786–9798
Zhou X, Feng Y, Zhan Z, Chen J (2014) Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem 289(42):28827–28834
Acknowledgments
The authors sincerely thank the Department of Science and Technology for providing INSPIRE fellowship (DST/INSPIRE Fellowship/2013/326).
Author information
Authors and Affiliations
Contributions
Dr. Gino A Kurian has designed the work and helped in data interpretation and writing the manuscript. Ms. Mahalakshmi A has executed the experiments, analyzed the data, interpreted the results, wrote the manuscript, and compiled the literature sources.
Corresponding author
Ethics declarations
All procedures for the handling of the animals during the investigations were reviewed and approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA Approval No. 300/SASTRA/IAEC/RPP), India.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Statement of significance
Hydrogen sulfide post-conditioning failed to recover cardiac mitochondrial function in diabetic cardiomyopathy rat heart from ischemia-reperfusion resulted injury.
Electronic supplementary material
ESM 1
(DOCX 1462 kb)
Rights and permissions
About this article
Cite this article
A, M., Kurian, G.A. Mitochondrial dysfunction plays a key role in the abrogation of cardioprotection by sodium hydrosulfide post-conditioning in diabetic cardiomyopathy rat heart. Naunyn-Schmiedeberg's Arch Pharmacol 393, 339–348 (2020). https://doi.org/10.1007/s00210-019-01733-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01733-z