Abstract
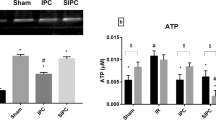
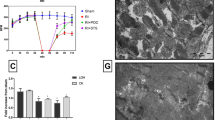
Cardiac mitochondrial dysfunction is considered to be the main manifestation in the pathology of ischemia reperfusion injury, and by restoring its functional activity, hydrogen sulfide (H2S), a novel endogenous gaseotransmitter renders cardioprotection. Given that interfibrillar (IFM) and subsarcolemmal (SSM) mitochondria are the two main types in the heart, the present study investigates the specific H2S-mediated action on IFM and SSM during ischemic reperfusion in the Langendorff rat heart model. Rats were randomly divided into five groups, namely normal, ischemic control, reperfusion control (I/R), ischemic post-conditioning (POC), and H2S post-conditioning (POC_H2S). In reperfusion control, cardiac contractility decreased, and lactate dehydrogenase, creatine kinase, and infracted size increased compared to both normal and ischemic group. In hearts post-conditioned with H2S and the classical method improved cardiac mechanical function and decreased cardiac markers in the perfusate and infarct size significantly. Both POC and POC_H2S exerts its cardioprotective effect of preserving the IFM, as evident by significant improvement in electron transport chain enzyme activities and mitochondrial respiration. The in vitro action of H2S on IFM and SSM from normal and I/R rat heart supports H2S and mediates cardioprotection via IFM preservation. Our study indicates that IFM play an important role in POC_H2S mediated cardioprotection from reperfusion injury.







Similar content being viewed by others
References
Andrew JT, Lindsay SB, Stanley BD, Corinne Z, William LH, Paul SB (2006) Mitochondrial dysfunction in cardiac ischemia–reperfusion injury: ROS from complex I, without inhibition. Biochim Biophys Acta (BBA) - Mol Basis Dis 1762(2):223–231
Ang AD, Konigstorfer A, Giles GI, Bhatia M (2012) Measuring free tissue sulfide. Adv Biol Chem 2:360–365
Biase FH, Franco MM, Goulart LR, Antunes RC (2002) Protocol for extraction of genomic DNA from swine solid tissues. Genet Mol Biol 25(3):3313–3315
Carden DL, Granger DN (2000) Pathophysiology of ischaemia-reperfusion injury. J Pathol 190(3):255–266
Chen YR, Chen CL, Pfeiffer DR, Zweier JL (2007) Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem 282:32640–32654
Chen CL, Chen J, Rawale S, Varadharaj S, Kaumaya PP, Zweier JL, Chen YR (2008) Protein tyrosine nitration of the flavin subunit is associated with oxidative modification of mitochondrial complex II in the post-ischemic myocardium. J Biol Chem 283:27991–28003
Chevion M, Jiang Y, Har-El R, Berenshtein E, Uretzky G, Kitrossky N (1993) Copper and iron are mobilized following myocardial ischemia: possible predictive criteria for tissue injury. Proc Natl Acad Sci U S A 90(3):1102–1106
Dudek M, Knutelska J, Bednarski M, Nowinski L, Zygmunt M, Bilska-Wilkosz A, Iciek M, Otto M, Zytka I, Sapa J, Włodek L, Filipek B (2014) Alpha lipoic acid protects the heart against myocardial post ischemia–reperfusion arrhythmias via KATP channel activation in isolated rat hearts. Pharmacol Rep 66(3):499–504
Emilie L, Sabria M, Mireille A, Catherine C, Francois B, Frederic B (2010) Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta (BBA) Bioenerg 1797(8):1500–1511
Frazier AE, Thorburn DR (2012) Biochemical analyses of the electron transport chain complexes by spectrophotometry Methods. Mol Biol 837:49–62
Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C (2004) H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun 313(2):362–368
Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F (2007) Sulfide, the first inorganic substrate for human cells. FASEB J 21(8):1699–1706
Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM (2008) Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol 587(1-3):1–7
John WE, John WC, Joanna M, Jeannette ED, David WK, Ling T, Xiangying J, Rosario S, Levente K, Csaba S, Hideo K, Chi-Wing C, David JL (2007) Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A 104(39):15560–15565
Jonathan PL, Chad KN, Hena A, Sana A, John WC (2014) Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med Gas Res 4:20
Katalin M, Ciro C, Katalin E, Andreas P, Szabo C (2013) Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J 27(2):601–611
Kurian GA, Sachu, Thomas V (2005) Effect of aqueous extract of Desmodium Gangeticum root in the severity of isoproterenol induced Myocardial infarcted rats. J Ethnapharmacol 97(3):457–461
Kurian GA, Berenshtein E, Saada A, Chevion M (2012a) Rat cardiac mitochondrial sub-population show distinct features of oxidative phosphorylation during ischemia, reperfusion and ischemic preconditioning. Cell Physiol Biochem 30(1):83–94
Kurian GA, Berenshtein E, Kakhlon O, Chevion M (2012b) Energy status determines the distinct biochemical and physiological behavior of interfibrillar and sub-sarcolemmal mitochondria. Biochem Biophys Res Commun 428(3):376–382
Lacerda L, Somers S, Opie LH, Lecour S (2009) Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res 84(2):201–208
Lee CH, Hwang JH, Lee YS, Cho KS (1995) Purification and characterization of mouse liver rhodanese. J Biochem Mol Biol 28:170–176
Lesnefsky EJ, Gudz T, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL (2001) Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys 385:117–128
Li H, Wang Y, Wei C, Bai S, Zhao Y, Li H, Wu B, Wang R, Wu L, Xu C (2015) Mediation of exogenous hydrogen sulfide in recovery of ischemic post-conditioning-induced cardioprotection via down-regulating oxidative stress and up-regulating PI3K/Akt/GSK-3β pathway in isolated aging rat hearts. Cell Biosci 5:11
Luan HF, Zhao ZB, Zhao QH, Zhu P, Xiu MY, Ji Y (2012) Hydrogen sulfide postconditioning protects isolated rat hearts against ischemia and reperfusion injury mediated by the JAK2/STAT3 survival pathway. Braz J Med Biol Res 45(10):898–905
Mailloux RJ, Jin X, Willmore WG (2014) Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol 2:123–139
Maria-Giulia P, Pasquale P, Claudia P (2011) Ischemia/reperfusion injury and cardioprotective mechanisms: role of mitochondria and reactive oxygen species. World J Cardiol 3(6):186–200
Martens ME, Chang CH, Lee CP (1986) Reye’s syndrome: mitochondrial swelling and Ca2+ release induced by Reye’s plasma, allantoin, and salicylate. Arch Biochem Biophys 244:773–786
Mensah K, Mocanu MM, Yellon DM (2005) Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: a potential role for phosphatase and tensin homolog deleted on chromosome ten. J Am Coll Cardiol 45(8):1287–1291
Michel O, Gary FB, Fabio DL, Peter F, David GD, Derek JH, Gerd H, Jakob VJ, Derek MY, Rainer S (2010) Postconditioning and protection from reperfusion injury: where do we stand? Cardiovasc Res 87(3):406–423
Ming F, Weihua Z, Lingyun W, Guangdong Y, Hongzhu L, Rui W (2012) Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A 109(8):2943–2948
Modis K, Bos EM, Calzia E, van Goor H, Coletta C, Papapetropoulos A, Mark RH, Peter R, Frederic B, Csaba S (2014) Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects. Br J Pharmacol 171(8):2123–2146
Palmer JW, Tandler B, Hoppel CL (1977) Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252(23):8731–8739
Pan TT, Neo KL, Hu LF, Yong QC, Bian JS (2008) H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am J Physiol Cell Physiol 294(1):C169–C177
Paul BD, Snyder SH (2012) H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13:499–507
Raymond WL, David WK, Jeannette ED (1996) Sulfide-stimulation of oxygen consumption rate and cytochrome reduction in gills of the estuarine mussel Geukensia demissa. Bid Bull 191:421–430
Scaduto RC, Grotyohann LW (1999) Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 76:469–477
Stepien PP, Pieniazek NJ (1973) Use of the L-serine sulfhydrylase assay for the estimation of cystathionine beta-synthase. Anal Biochem 54:294–299
Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, Bouillaud F (2014) Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol 171(8):2099–2122
Tatjana MH (2011) Modulation of sulfide oxidation and toxicity in rat mitochondria by dehydroascorbic acid. Biochim Biophys Acta (BBA) Bioenerg 1807(9):1206–1213
Yeong-Renn C, Jay L (2014) Zweier2 cardiac mitochondria and ROS generation. Circ Res 114(3):524–537
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banu, S.A., Ravindran, S. & Kurian, G.A. Hydrogen sulfide post-conditioning preserves interfibrillar mitochondria of rat heart during ischemia reperfusion injury. Cell Stress and Chaperones 21, 571–582 (2016). https://doi.org/10.1007/s12192-016-0682-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-016-0682-8