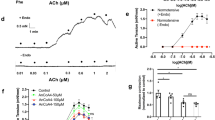
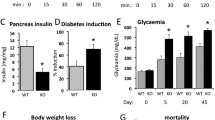
Abstract
Diabetes mellitus comprises a heterogeneous group of metabolic disorders with underlying hyperglycemia and secondary cardiovascular complications. Growing evidence suggests that vascular dysfunction is among the most important causes of diabetic cardiovascular disease. Therefore, we determined whether streptozotocin (STZ)-induced diabetes in mice affects blood pressure and cerebral arterial responsiveness to angiotensin (Ang) II and acetylcholine (ACh), which are important modulators of cerebrovascular autoregulation. Diabetes was induced using a single intraperitoneal injection of STZ (50 mg/kg). Blood pressure was measured in conscious mice using the indirect tail-cuff method. Functional studies of the isolated arteries’ response to vasoactive substances were performed using a micro-organ-bath system at 60 days after STZ injection. Systolic, diastolic, and mean blood pressures significantly increased at days 45 and 60 in the STZ-induced diabetic mice. In the isolated basilar arteries, ACh-induced relaxation, which is dependent on nitric oxide (NO) production from endothelial cells, decreased. In contrast, Ang II-induced contraction, mediated via rho-kinase activation in the smooth muscle, increased in the diabetic mice. There was significantly greater relaxation in the precontracted isolated basilar arteries of diabetic mice that had been treated with Y27632, a rho-kinase inhibitor, than in the control mice arteries. Pretreatment with Nω-nitro-l-arginine (L-NAME), an NO synthase inhibitor, significantly enhanced Ang II-induced contraction and Y27632-induced relaxation in the control basilar arteries but not in the STZ-induced diabetic mice arteries. These results suggest that decreased NO bioavailability and enhanced rho-kinase activity in basilar arteries contribute to altered reactivity to ACh and Ang II, respectively, in STZ-induced diabetic mice.







Similar content being viewed by others
References
Adel H, Taye A, Khalifa MM (2014) Spironolactone improves endothelial dysfunction in streptozotocin-induced diabetic rats. Naunyn Schmiedeberg’s Arch Pharmacol 387:1187–1197. doi:10.1007/s00210-014-1048-3
Arrick DM, Sharpe GM, Sun H, Mayhan WG (2008) Losartan improves impaired nitric oxide synthase-dependent dilation of cerebral arterioles in type 1 diabetic rats. Brain Res 1209:128–135. doi:10.1016/j.brainres.2008.03.020
Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G (2005) Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol 25:1610–1616. doi:10.1161/01.ATV.0000172688.26838.9f
Bagi Z, Feher A, Cassuto J, Akula K, Labinskyy N, Kaley G, Koller A (2011) Increased availability of angiotensin AT1 receptors leads to sustained arterial constriction to angiotensin II in diabetes—role for rho-kinase activation. Br J Pharmacol 163:1059–1068. doi:10.1111/j.1476-5381.2011.01307.x
Besnard S, Bakouche J, Lemaigre-Dubreuil Y, Mariani J, Tedgui A, Henrion D (2002) Smooth muscle dysfunction in resistance arteries of the staggerer mouse, a mutant of the nuclear receptor RORα. Circ Res 90:820–825. doi:10.1161/01.RES.0000014489.24705.71
Boels PJ, Troschka M, Rüegg JC, Pfitzer G (1991) Higher Ca2+ sensitivity of triton-skinned guinea pig mesenteric microarteries as compared with large arteries. Circ Res 69:989–996. doi:10.1161/01.RES.69.4.989
Chen B, Zhao Q, Ni R, Tang F, Shan L, Cepinskas I, Cepinskas G, Wang W, Schiller PW, Peng T (2014) Inhibition of calpain reduces oxidative stress and attenuates endothelial dysfunction in diabetes. Cardiovasc Diabetol 13:88. doi:10.1186/1475-2840-13-88
Chitaley K, Webb RC (2002) Nitric oxide induces dilation of rat aorta via inhibition of rho-kinase signaling. Hypertension 39:438–442. doi:10.1161/hy02t2.102960
Christlieb AR, Janka HU, Kraus B, Gleason RE, Icasas-Cabral EA, Aiello LM, Cabral BV, Solano A (1976) Vascular reactivity to angiotensin II and to norepinephrine in diabetic subjects. Diabetes 25:268–274. doi:10.2337/diab.25.4.268
Didion SP, Lynch CM, Faraci FM (2007) Cerebral vascular dysfunction in TallyHO mice: a new model of type II diabetes. Am J Physiol Heart Circ Physiol 292:1579–1583. doi:10.1152/ajpheart.00939.2006
Didion SP, Lynch CM, Baumbach GL, Faraci FM (2005) Impaired endothelium-dependent responses and enhanced influence of rho-kinase in cerebral arterioles in type II diabetes. Stroke 36:342–347. doi:10.1161/01.STR.0000152952.42730.92
El-Remessy AB, Tawfik HE, Matragoon S, Pillai B, Caldwell RB, Caldwell RW (2010) Peroxynitrite mediates diabetes-induced endothelial dysfunction: possible role of rho kinase activation. Exp Diabetes Res 2010:247861. doi:10.1155/2010/247861
Faraci FM, Lamping KG, Modrick ML, Ryan MJ, Sigmund CD, Didion SP (2006) Cerebral vascular effects of angiotensin II: new insights from genetic models. J Cereb Blood Flow Metab 26:449–455. doi:10.1038/sj.jcbfm.9600204
Ferrannini E, Cushman WC (2012) Diabetes and hypertension: the bad companions. Lancet 380:601–610. doi:10.1016/S0140-6736(12)60987-8
Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, Gong MC (2005) COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res 67:723–735. doi:10.1016/j.cardiores.2005.04.008
Hadi HA, Suwaidi JA (2007) Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 3:853–876
Haidara MA, Yassin HZ, Rateb M, Ammar H, Zorkani MA (2006) Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr Vasc Pharmaco l4:215–227. doi:10.2174/157016106777698469
Hassan N, El-Bassossy HM, Zakaria MN (2013) Heme oxygenase-1 induction protects against hypertension associated with diabetes: effect on exaggerated vascular contractility. Naunyn Schmiedeberg's Arch Pharmacol 386:217–226. doi:10.1007/s00210-012-0822-3
Hill MA, Larkins RG (1989) Altered microvascular reactivity in streptozotocin-induced diabetes in rats. Am J Phys 257:1438–1445
Islam MZ, Van Dao C, Shiraishi M, Miyamoto A (2016) Methylmercury affects cerebrovascular reactivity to angiotensin II and acetylcholine via rho-kinase and nitric oxide pathways in mice. Life Sci 147:30–38. doi:10.1016/j.lfs.2016.01.033
Islam MZ, Watanabe Y, Nguyen HT, Yamazaki-Himeno E, Obi T, Shiraishi M, Miyamoto A (2014) Vasomotor effects of acetylcholine, bradykinin, noradrenaline, 5-hydroxytryptamine, histamine and angiotensin II on the mouse basilar artery. J Vet Med Sci 76:1339–1345. doi:10.1292/jvms.14-0223
Kobayashi T, Oishi K, Hayashi Y, Matsumoto T, Kamata K (2006) Changes in aortic endothelial gene expression and relaxation responses following chronic short-term insulin treatment in diabetic rats. Atherosclerosis 185:47–57. doi:10.1016/j.atherosclerosis.2005.05.026
Kovacic JC, Castellano JM, Farkouh ME, Fuster V (2014) The relationships between cardiovascular disease and diabetes: focus on pathogenesis. Endocrinol Metab Clin N Am 43:41–57. doi:10.1016/j.ecl.2013.09.007
Laakso M, Kuusisto J (2014) Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 10:293–302. doi:10.1038/nrendo.2014.29
Leo CH, Hart JL, Woodman OL (2011) 3′,4′-Dihydroxyflavonol restores endothelium-dependent relaxation in small mesenteric artery from rats with type 1 and type 2 diabetes. Eur J Pharmacol 659:193–198. doi:10.1016/j.ejphar.2011.03.018
Loirand G, Guerin P, Pacaud P (2006) Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 98:322–334. doi:10.1161/01.RES.0000201960.04223.3c
Mayhan WG, Arrick DM (2017) Tetrahydrobiopterin rescues impaired responses of cerebral resistance arterioles during type 1 diabetes. Diab Vasc Dis Res 14:33–39. doi:10.1177/1479164116675490
McNally PG, Watt PA, Rimmer T, Burden AC, Hearnshaw JR, Thurston H (1994) Impaired contraction and endothelium-dependent relaxation in isolated resistance vessels from patients with insulin-dependent diabetes diabetes mellitus. Clin Sci (Lond) 87:31–36
Merlini M, Shi Y, Keller S, Savarese G, Akhmedov A, Derungs R, Spescha RD, Kulic L, Nitsch RM, Lüscher TF, Camici GG (2016) Reduced nitric oxide bioavailability mediates cerebroarterial dysfunction independent of cerebral amyloid angiopathy in a mouse model of Alzheimer’s disease. Am J Physiol Heart Circ Physiol doi. doi:10.1152/ajpheart.00607.2016
Miike T, Kunishiro K, Kanda M, Azukizawa S, Kurahashi K, Shirahase H (2008) Impairment of endothelium-dependent ACh-induced relaxation in aorta of diabetic db/db mice—possible dysfunction of receptor and/or receptor-G protein coupling. Naunyn Schmiedeberg’s Arch Pharmacol 377:401–410. doi:10.1007/s00210-008-0261-3
Miller AA, Drummond GR, De Silva TM, Mast AE, Hickey H, Williams JP, Broughton BR, Sobey CG (2009) NADPH oxidase activity is higher in cerebral versus systemic arteries of four animal species: role of Nox2. Am J Physiol Heart Circ Physiol 296:220–225. doi:10.1152/ajpheart.00987.2008
Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z (2002) Rho GTPase/rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol 22:8467–8477. doi:10.1128/MCB.22.24.8467-8477.2002
Miyamoto A, Hashiguchi Y, Obi T, Ishiguro S, Nishio A (2007) Ibuprofen or ozagrel increases NO release and L-nitro arginine induces TXA2 release from cultured porcine basilar arterial endothelial cells. Vasc Pharmacol 46:85–90. doi:10.1016/j.vph.2006.06.018
Nacci C, Tarquinio M, De Benedictis L, Mauro A, Zigrino A, Carratù MR, Quon MJ, Montagnani M (2009) Endothelial dysfunction in mice with streptozotocin-induced type 1 diabetes is opposed by compensatory over expression of cyclooxygenase-2 in the vasculature. Endocrinology 150:849–861. doi:10.1210/en.2008-1069
Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM (2013) Interleukin-17 causes rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res 97:696–704. doi:10.1093/cvr/cvs422
Ning R, Chopp M, Yan T, Zacharek A, Zhang C, Roberts C, Cui X, Lu M, Chen J (2012) Tissue plasminogen activator treatment of stroke in type-1 diabetes rats. Neuroscience 222:326–332. doi:10.1016/j.neuroscience.2012.07.018
Nishimura Y (1996) Characterization of 5-hydroxytryptamine receptors mediating contractions in basilar arteries from stroke-prone spontaneously hypertensive rats. Br J Pharmacol 117:1325–1133
Nobe K, Hashimoto T, Honda K (2012) Two distinct dysfunction in diabetic mouse mesenteric artery contraction are caused by changes in the RhoA-rho kinase signaling pathway. Eur J Pharmacol 683:217–225. doi:10.1016/j.ejphar.2012.03.022
Pernomian L, Santos Gomes M, Baraldi Araujo Restini C, Naira Zambelli Ramalho L, Renato Tirapelli C, Maria de Oliveira A (2012) The role of reactive oxygen species in the modulation of the contraction induced by angiotensin II in carotid artery from diabetic rat. Eur J Pharmacol 678:15–25. doi:10.1016/j.ejphar.2011.12.036
Pieper GM (1999) Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: importance of disease duration. Diabetologia 42:204–213. doi:10.1007/s001250051140
Pinho JF, Medeiros MA, Capettini LS, Rezende BA, Campos PP, Andrade SP, Cortes SF, Cruz JS, Lemos VS (2010) Phosphatidylinositol 3-kinase-δ up-regulates L-type Ca2+ currents and increases vascular contractility in a mouse model of type 1 diabetes. Br J Pharmacol 161:1458–1471. doi:10.1111/j.1476-5381.2010.00955.x
Rikitake Y, Liao JK (2005) Rho GTPases, statins, and nitric oxide. Circ Res 97:1232–1235. doi:10.1161/01.RES.0000196564.18314.23
Sarubbi D, McGiff JC, Quilley J (1989) Renal vascular responses and eicosanoid release in diabetic rats. Am J Phys 257:762–768
Schram MT, Chaturvedi C, Schalkwijk C, Giorgino F, Ebeling P, Fuller JH, Stehouwer CD (2003) Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes: the EURODIAB prospective complications study. Diabetes Care 26:2165–2173. doi:10.2337/diacare.26.7.2165
Sonobe T, Tsuchimochi H, Schwenke DO, Pearson JT, Shirai M (2015) Treadmill running improves hindlimb arteriolar endothelial function in type 1 diabetic mice as visualized by X-ray microangiography. Cardiovasc Diabetol 14:51. doi:10.1186/s12933-015-0217-0
Tang EH, Vanhoutte PM (2010) Endothelial dysfunction: a strategic target in the treatment of hypertension? Pflugers Arch 459:995–1004. doi:10.1007/s00424-010-0786-4
Viswanad B, Srinivasan K, Kaul CL, Ramarao P (2006) Effect of tempol on altered angiotensin II and acetylcholine-mediated vascular responses in thoracic aorta isolated from rats with insulin resistance. Pharmacol Res 53:209–215. doi:10.1016/j.phrs.2005.11.002
Xie Z, Gong MC, Su W, Xie D, Turk J, Guo Z (2010) Role of calcium-independent phospholipase A2β in high glucose-induced activation of RhoA, rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hypercontractility in diabetic animals. J Biol Chem 285:8628–8638. doi:10.1074/jbc.M109.057711
Yao L, Chandra S, Toque HA, Bhatta A, Rojas M, Caldwell RB, Caldwell RW (2013) Prevention of diabetes-induced arginase activation and vascular dysfunction by rho kinase (ROCK) knockout. Cardiovasc Res 97:509–519. doi:10.1093/cvr/cvs371
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (no. 26450407) from the Japan Society for the Promotion of Sciences (JSPS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary materials
ESM 1
Effect of streptozotocin (STZ) treatment on blood pressure and heart rate. Systolic [a], diastolic [b], and mean [c] blood pressure, and heart rate [d] were measured every 15 days in conscious mice using the indirect tail-cuff method at 30 days after injection of STZ or vehicle. Results are expressed as the mean ± SEM (n = 11/group). (TIFF 1180 kb)
ESM 2
Effect of streptozotocin (STZ) treatment on blood glucose levels (a), and on basilar arterial responsiveness to acetylcholine (ACh) (b), angiotensin (Ang) II (c), and Y27632 (d) in mice. Mice received a single intraperitoneal injection of STZ (50 mg/kg) or vehicle (0.1 M citrate buffer solution) after an overnight fast. Blood glucose levels and basilar artery response to ACh, Ang II, and Y27632 were measured 30 days after the injection. Results are expressed as the mean ± SEM (n = 5-8/group). (TIFF 1181 kb)
Rights and permissions
About this article
Cite this article
Islam, M.Z., Van Dao, C., Miyamoto, A. et al. Rho-kinase and the nitric oxide pathway modulate basilar arterial reactivity to acetylcholine and angiotensin II in streptozotocin-induced diabetic mice. Naunyn-Schmiedeberg's Arch Pharmacol 390, 929–938 (2017). https://doi.org/10.1007/s00210-017-1396-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-017-1396-x