Abstract
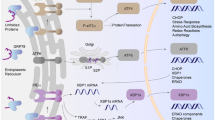
Endothelium-dependent contraction (EDC) exists in blood vessels of normotensive animals, but is exaggerated in hypertension. An early signal in EDC is cytosolic Ca2+ rise in endothelial cells. In this study we investigated the functional role of Orai1, a major endothelial cell Ca2+ entry channel, in EDC. Hypertension model was established in WT mice by intake of L-NNA in the drinking water (0.5 g/L) for 4 weeks or osmotic pump delivery of Ang II (1.5 mg·kg−1·d−1) for 2 weeks. In TRPC5 KO mice, the concentration of L-NNA and Ang II were increased to 1 g/L or 2 mg·kg−1·d−1, respectively. Arterial segments were prepared from carotid arteries and aortas, and EDC was elicited by acetylcholine in the presence of Nω-nitro-L-arginine methyl ester. We showed that low concentration of acetylcholine (3–30 nM) initiated relaxation in phenylephrine-precontracted carotid arteries of both normotensive and hypertensive mice, while high concentration of acetylcholine (0.1–2 μM) induced contraction. Application of selective Orai1 inhibitors AnCoA4 (100 μM) or YM58483 (400 nM) had no effect on ACh-induced relaxation but markedly reduced acetylcholine-induced EDC. We found that EDC was increased in hypertensive mice compared with that of normotensive mice, which was associated with increased Orai1 expression in endothelial cells of hypertensive mice. Compared to TRPC5 and TRPV4, which were also involved in EDC, endothelial cell Orai1 had relatively greater contribution to EDC than either TRPC5 or TRPV4 alone. We identified COX-2, followed by PGF2α, PGD2 and PGE2 as the downstream signals of Orai1/TRPC5/TRPV4. In conclusion, Orai1 coordinates together with TRPC5 and TRPV4 in endothelial cells to regulate EDC responses. This study demonstrates a novel function of Orai1 in EDC in both normotensive and hypertensive mice, thus providing a general scheme about the control of EDC by Ca2+-permeable channels.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Wang L, Cheng CK, Yi M, Lui KO, Huang Y. Targeting endothelial dysfunction and inflammation. J Mol Cell Cardiol. 2022;168:58–67.
Zhao S, Cheng CK, Zhang CL, Huang Y. Interplay between oxidative stress, cyclooxygenases, and prostanoids in cardiovascular diseases. Antioxid Redox Signal. 2021;34:784–99.
Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol Oxf Engl. 2009;196:193–222.
Wong MS, Vanhoutte PM. COX-mediated endothelium-dependent contractions: from the past to recent discoveries. Acta Pharmacol Sin. 2010;31:1095–102.
Félétou M, Huang Y, Vanhoutte PM. Vasoconstrictor prostanoids. Pflug Arch. 2010;459:941–50.
Félétou M, Vanhoutte PM, Verbeuren TJ. The thromboxane/endoperoxide receptor (TP): the common villain. J Cardiovasc Pharmacol. 2010;55:317–32.
Liang C, Zhang YT, Zhuo D, Lo CY, Yu LB, Lau CW, et al. Endothelial cell transient receptor potential channel C5 (TRPC5) is essential for endothelium-dependent contraction in mouse carotid arteries. Biochem Pharmacol. 2019;159:11–24.
Zhang P, Sun C, Li H, Tang C, Kan H, Yang Z, et al. TRPV4 (Transient Receptor Potential Vanilloid 4) mediates endothelium-dependent contractions in the aortas of hypertensive mice. Hypertens Dallas Tex 1979. 2018;71:134–42.
Chu Y, Wang S, Zhu Y, Yu F, Zhang K, Ma X. TRPC5 mediates endothelium-dependent contraction in the carotid artery of diet-induced obese mice. Hypertens Res. 2022;45:1945–53.
Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015;95:1383–436.
Lacruz RS, Feske S. Diseases caused by mutations in ORAI1 and STIM1. Ann N Y Acad Sci. 2015;1356:45–79.
Beech DJ. Orai1 calcium channels in the vasculature. Pflug Arch. 2012;463:635–47.
Ruhle B, Trebak M. Emerging roles for native Orai Ca2+ channels in cardiovascular disease. Curr Top Membr. 2013;71:209–35.
Moccia F, Negri S, Shekha M, Faris P, Guerra G. Endothelial Ca2+ signaling, angiogenesis and vasculogenesis: just what it takes to make a blood vessel. Int J Mol Sci. 2019;20:3962.
Ma X, Du J, Zhang P, Deng J, Liu J, Lam FF, et al. Functional role of TRPV4-KCa2.3 signaling in vascular endothelial cells in normal and streptozotocin-induced diabetic rats. Hypertens Dallas Tex 1979. 2013;62:134–9.
Sadaghiani AM, Lee SM, Odegaard JI, Leveson-Gower DB, McPherson OM, Novick P, et al. Identification of Orai1 channel inhibitors by using minimal functional domains to screen small molecule microarrays. Chem Biol. 2014;21:1278–92.
Ishikawa J, Ohga K, Yoshino T, Takezawa R, Ichikawa A, Kubota H, et al. A pyrazole derivative, YM-58483, potently inhibits store-operated sustained Ca2+ influx and IL-2 production in T lymphocytes. J Immunol Balt Md 1950. 2003;170:4441–9.
Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–58.
Tang EH, Vanhoutte PM. Endothelial dysfunction: a strategic target in the treatment of hypertension? Pflug Arch. 2010;459:995–1004.
Just S, Chenard BL, Ceci A, Strassmaier T, Chong JA, Blair NT, et al. Treatment with HC-070, a potent inhibitor of TRPC4 and TRPC5, leads to anxiolytic and antidepressant effects in mice. PLoS ONE. 2018;13:e0191225.
Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA. 2010;107:19084–9.
Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–99.
Domínguez-Rodríguez A, Díaz I, Rodríguez-Moyano M, Calderón-Sánchez E, Rosado JA, Ordóñez A, et al. Urotensin-II signaling mechanism in rat coronary artery: role of STIM1 and Orai1-dependent store operated calcium influx in vasoconstriction. Arterioscler Thromb Vasc Biol. 2012;32:1325–32.
Ávila-Medina J, Calderón-Sánchez E, González-Rodríguez P, Monje-Quiroga F, Rosado JA, Castellano A, et al. Orai1 and TRPC1 proteins co-localize with CaV1.2 channels to form a signal complex in vascular smooth muscle cells. J Biol Chem. 2016;291:21148–59.
Chen M, Li J, Jiang F, Fu J, Xia X, Du J, et al. Orai1 forms a signal complex with BKCa channel in mesenteric artery smooth muscle cells. Physiol Rep. 2016;4:e12682.
Trebak M. STIM/Orai signalling complexes in vascular smooth muscle. J Physiol. 2012;590:4201–8.
Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295:C779–790.
Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, et al. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23:2425–37.
Acknowledgements
This work was supported by grants from Hong Kong Research Grant Committee (14100619, RIF/R4005-18F) and Innovation and Technology Fund ITS/212/21.
Author information
Authors and Affiliations
Contributions
XQY revised the manuscript, supervised the study, and obtained funding; XL contributed to the experiment design, conducted the experiments, performed data analyzes, prepared the figures, and drafted the manuscript. ZCL, CYL, TYJ, and CWL conducted the experiments. All authors contributed to the final manuscript and approved submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals issued by the US National Institute of Health (NIH) and approved by the Animal Experimentation Ethics Committee, Chinese University of Hong Kong.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Lei, Zc., Lo, C.Y. et al. Endothelial cell Orai1 is essential for endothelium-dependent contraction of mouse carotid arteries in normotensive and hypertensive mice. Acta Pharmacol Sin 45, 975–987 (2024). https://doi.org/10.1038/s41401-024-01227-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-024-01227-6
- Springer Nature Singapore Pte Ltd.