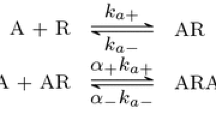Abstract
Bivalent ligands often display high affinity/avidity for and long residence time at their target. Thereto responsible is the synergy that emanates from the simultaneous binding of their two pharmacophores to their respective target sites. Thermodynamic cycle models permit the most complete description of the binding process, and thereto, corresponding differential equation-based simulations link the “microscopic” rate constants that govern the individual binding steps to the “macroscopic” bivalent ligand’s binding properties. Present simulations of heterobivalent ligand binding led to an appreciably simpler description thereof. The thermodynamic cycle model can be split into two pathways/lanes that the bivalent ligand can solicit to reach fully bound state. Since the first binding event prompts the still free pharmacophore to stay into “forced proximity” of its target site, such lanes can be looked into by the equations that also apply to induced fit binding mechanisms. Interestingly, the simplest equations apply when bivalency goes along with a large gain in avidity. The overall bivalent ligand association and dissociation will be swifter than via each lane apart, but it is the lane that allows the fastest bidirectional “transit” between the free and the fully bound target that is chiefly solicited. The bivalent ligand’s residence time is governed not only by the stability of the fully bound complex but also by the ability of freshly dissociated pharmacophores to successfully rebind. Hence, the presence of a slow-associating pharmacophore could be counterproductive. Yet, a long residence time is unfortunately also responsible for the slow attainment of binding equilibrium.







Similar content being viewed by others
References
Charlton S, Vauquelin G (2010) Elusive equilibrium: the challenge of interpreting receptor pharmacology using calcium assays. Brit J Pharmacol 161:1250–1265
Christensen LLH (1997) Theoretical analysis of protein concentration determination using biosensor technology under conditions of partial mass transport limitation. Anal Bioch 249:153–164
Christopoulos A (2002) Allosteric binding sites on cell-surface receptors, novel targets for drug discovery. Nat Rev Drug Discov 1:198–210
Christopoulos A, Kenakin T (2002) G protein-coupled receptor allosterism and complexing. Pharmacol Rev 54:323–374
Coombs D, Goldstein B (2004) Effects of geometry of the immunological synapse on the delivery of effector molecules. Biophys J 87:2215–2220
Copeland RA (2010) The dynamics of drug-target interactions: drug-target residence time and its impact on efficacy and safety. Expert Opin Drug Discov 5:305–510
Copeland RA (2011) Conformational adaptation of drug–target interactions and residence time. Future Med Chem 3:1491–1501
Copeland RA, Pompliano DL, Meek TD (2006) Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov 5:730–739
Daum S, Lücke C, Wildeman D, Schiener-Fischrer C (2007) On the benefit of bivalency in peptide ligand/Pin1 interactions. J Mol Biol 374:147–161
De Meyts P, Gauguin L, Manegold Svendsen A, Sarhan M, Knudsen L, Nohr J et al (2009) Ann N Y Acad Sci 160:45–53
DeLisi C (1981) The effect of cell size and receptor density on ligand-receptor reaction rate constants. Mol Immunol 18:507–511
DeLisi C, Wiegel FW (1981) Effect of nonspecific forces and finite receptor number on rate constants of ligand–cell bound–receptor interactions. Proc Natl Acad Sci USA 78:5569–5572
Goldstein B, Dembo M (1995) Approximating the effects of diffusion on reversible reactions at the cell surface: ligand-receptor kinetics. Biophys J 68:1222–1230
Goldstein B, Posner RG, Torney DC, Erickson J, Holowka D, Baird B (1989) Competition between solution and cell surface receptors for ligand. Dissociation of hapten bound to surface antibody in the presence of solution antibody. Biophys J 56:955–966
Guo D, Mulder-Krieger T, IJzerman AP, Heitman LH (2012) Functional efficacy of adenosine A2A receptor agonists is positively correlated to their receptor residence time. Brit J Pharmacol 166:1846–1959
Hoare SRJ (2007) Allosteric modulators of class B G-protein-coupled receptors. Curr Neuropharmacol 5:168–179
Holliger P, Hudson PJ (2005) Engineered antibody fragments and the rise of single domains. Nat Biotechnol 23:1126–1136
Hudson PJ, Kortt AA (1999) High avidity scFv multimers; diabodies and triabodies. J Immunol Meth 231:177–189
Kamal M, Jockers R (2009) Bitopic ligands, all-in-one orthostetic and allosteric. F1000 Biol Reports 1, 77. doi: 10.3410/B1-77
Kaufman EN, Jain RK (1992) Effect of bivalent interaction upon apparent antibody affinity: experimental confirmation of a theory using fluorescence photobleaching and implications for antibody binding assays. Cancer Res 52:4157–4167
Kramer RH, Karpen JW (1998) Spanning biding sites on allosteric proteins with polymer-linked ligand dimers. Nature 395:710–713
Lane JR, Sexton PM, Christopoulos A (2013) Bridging the gap: bitopic ligands of G-protein-coupled receptors. Trends Pharmacol Sci 34:59–65
Lefkowitz RJ, Pierce KL, Luttrell LM (2002) Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol Pharmacol 62:971–974
Lu H, Tonge PJ (2010) Drug–target residence time: critical information for lead optimization. Curr Opin Chem Biol 14:467–474
Luttrell LM, Lefkowitz RJ (2002) The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115:455–465
Mammen M, Choi W-K, Whitesides GM (1998) Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int 37:2754–2794
Mohr K, Tränkle C, Kostenis E, Barocelli E, De Amici M, Holzgrabe U (2010) Rational design of dualsteric GPCR ligands: quests and promise. Br J Pharmacol 159:997–1008
Morrison JF (1982) The slow-binding and slow, tight-binding inhibition of enzyme-catalysed reactions. Trends Biochem Sci 7:102–105
Müller KM, Arndt KM, Plückthun A (1998) Model and simulation of multivalent binding to fixed ligands. Anal Biochem 261:149–158
Núñez S, Venhorst J, Kruse CG (2012) Target–drug interactions: first principles and their application to drug discovery. Drug Discov Today 17:10–22
Perry DC, Mullis KB, Øie S, Sadée W (1980) Opiate antagonist receptor binding in vivo:evidence for a new receptor binding model. Brain Res 199:49–61
Plückthun A, Pack P (1997) New protein engineering approaches to multivalent and bispecific antibody fragments. Immunotechnol 3:83–105
Rudnick SI, Adams GP (2009) Affinity and avidity an antibody-based tumor targeting. Cancer Biother Radiopharm 24:155–161
Schuck P, Minton AP (1996) Analysis of mass transport-limited binding kinetics in evanescent wave biosensors. Anal Bioch 240:262–272
Steinfeld T, Mammen M, Smith JA, Wilson RD, Jasper JR (2007) A novel multivalent ligand that bridges the allosteric and orthosteric binding sites of the M2 muscarinic receptor. Mol Pharmacol 72:291–302
Strickland S, Palmer G, Massey V (1975) Determination of dissociation constants and specific rate constants of enzyme–substrate (or protein–ligand) interactions from rapid reaction kinetic data. J Biol Chem 250:4048–4052
Swinney DC (2004) Biochemical mechanisms of drug action: what does it take for success? Nat Rev Drug Discov 3:801–808
Swinney DC (2006) Can binding kinetics translate to a clinically differentiated drug? From theory to practice. Lett Drug Des Discov 3:569–574
Swinney DC (2009) The role of binding kinetics in therapeutically useful drug action. Curr Opin Drug Discov Devel 12:31–39
Sykes DA, Dowling MR, Charlton CJ (2009) Exploring the mechanism of agonist befficacy: a relationship between efficacy and agonist dissociation rate at the muscarinic M3 receptor. Mol Pharmacol 76:543–551
Szczuka A, Packeu A, Wennerberg M, Vauquelin G (2009) Molecular mechanism of the persistent bronchodilatory effect of the partial β2-adrenoceptor agonist salmeterol. Br J Pharmacol 158:183–194
Tummino PJ, Copeland RA (2008) Residence time of receptor-ligand complexes and its effect on biological function. Biochemistry 47:5481–5492
Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C et al (2005) Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J 24:1954–1964
Valant C, Lane JR, Sexton PM, Christopoulos A (2012) The best of two worlds? Bitopic orthostetic/allosteric ligands of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 52:153–178
Vauquelin G (2010) Rebinding, or why drugs may act longer in vivo than expected from their in vitro target residence time. Expert Opin Drug Discov 5:927–941
Vauquelin G, Charlton S (2010) Long-lasting target binding and rebinding as mechanisms to prolong in vivo drug action. Brit J Pharmacol 161:488–508
Vauquelin G, Charlton S (2013) Exploring avidity: understanding the potential gains in functional affinity and target residence time of bivalent and heterobivalent ligands. Brit J Pharmacol doi:10.1111/bph.12106
Vauquelin G, Van Liefde I (2006) From slow antagonist dissociation to long-lasting receptor protection. Trends Pharmacol Sci 27:355–359
Vauquelin G, Van Liefde I (2012) Radioligand dissociation measurements: potential interference of rebinding and allosteric mechanisms and physiological relevance of different model systems. Expert Opin Drug Discov 7:583–595
Vauquelin G, Morsing P, Fierens FLP, De Backer J-P, Vanderheyden PML (2001) A two-state receptor model for the interaction between angiotensin II AT1 receptors and their non-peptide antagonists. Biochem Pharmacol 61:277–284
Vauquelin G, Bostoen S, Vanderheyden P, Seeman P (2012) Clozapine, atypical antipsychotics and the benefits of fast-off D2 dopamine receptor antagonism. Naunyn-Schmiedeberg’s Arch Pharmacol 385:337–372
Weber M, Bujotzek A, Haag R (2012) Quantifying the rebinding effect in multivalent chemical ligand-receptor systems. J Chem Phys 137. doi: 054111–1–054111–10
Yu EW, Koshland DE (2001) Propagating conformational changes over long (and short) distances in proteins. Proc Natl Acad Sci USA 98:9517–9520
Zhang R, Monsma F (2009) The importance of drug-target residence time. Curr Opin Drug Discov Devel 12:488–496
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vauquelin, G. Simplified models for heterobivalent ligand binding: when are they applicable and which are the factors that affect their target residence time. Naunyn-Schmiedeberg's Arch Pharmacol 386, 949–962 (2013). https://doi.org/10.1007/s00210-013-0881-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-013-0881-0