Abstract
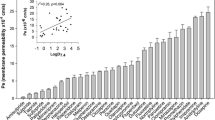
The muscarinic antagonists oxybutynin and trospium are used as spasmolytic agents for the treatment of overactive urinary bladder disease. Recently, it has been shown that trospium, but not oxybutynin, is a substrate of the multidrug efflux carrier P-glycoprotein, but carrier-mediated drug uptake has not been directly analysed for both drugs. However, trospium has been previously shown to exhibit inhibitory potency for the organic cation transporters (OCTs). The aim of the present study was to examine whether trospium and oxybutynin are substrates, i.e. are transported by the human OCTs (hOCT1, hOCT2 and hOCT3). Therefore, we measured total and specific (decynium-22-sensitive) uptake, and saturation kinetics of the uptake for [3H]oxybutynin and [3H]trospium in human embryonic kidney (HEK293) cells transiently transfected with the cDNA of hOCT1, hOCT2 or hOCT3. In addition, we determined IC50 values for inhibition of hOCT-mediated [3H]MPP+ uptake by unlabelled trospium and oxybutynin. Total uptake of [3H]oxybutynin was very high in all transfected HEK293 cells and only a small portion was due to specific, decynium-22-sensitive hOCT-mediated uptake. Oxybutynin inhibited [3H]MPP+ uptake by the three hOCTs with IC50 values between 20 and 130 μM. Direct determination of transport kinetics was measurable only at hOCT1 with K m of 8 μM and V max of 484 pmol/mg protein/min. The rank order of affinity (1/IC50 or 1/K m) of specific oxybutynin uptake was hOCT1 > hOCT2 = hOCT3. The observed high non-specific uptake is obviously a consequence of the high lipophilicity of this uncharged drug. Thus, hOCTs may not play a significant role for the overall pharmacokinetics and tissue distribution of oxybutynin. However, and in contrast to oxybutynin, uptake of [3H]trospium, an organic cation, was mainly due to carrier-mediated uptake by the three hOCTs. With IC50 values of 18, 1.4 and 710 μM (at hOCT1, hOCT2 and hOCT3, respectively) and K m values of 17 and 8 μM and about identical V max values of about 90 pmol/mg protein/min at hOCT1 and hOCT2, respectively; the rank order of affinity (1/IC50 or 1/K m) of specific uptake of trospium was hOCT2 > hOCT1 > > hOCT3. Thus, hOCTs very probably contribute to the active tubular and hepatobiliary secretion of trospium. Furthermore, hOCT1 and hOCT3 may be involved in the tissue uptake of this drug in the urinary bladder.


Similar content being viewed by others
References
Amend B, Hennenlotter J, Schafer T, Horstmann M, Stenzl A, Sievert KD (2008) Effective treatment of neurogenic detrusor dysfunction by combined high-dosed antimuscarinics without increased side-effects. Eur Urol 53:1021–1028
Biastre K, Burnakis T (2009) Trospium chloride treatment of overactive bladder. Ann Pharmacother 43:283–295
Choi M-K, Song I-S (2008) Organic cation transporters and their pharmacokinetic and pharmacodynamic consequences. Drug Metab Pharmacokinet 23:243–253
Diefenbach K, Donath F, Maurer A, Quispe Bravo S, Wernecke KD, Schwantes U, Haselmann J, Roots I (2003) Randomised, double-blind study of the effects of oxybutynin, tolterodine, trospium chloride and placebo on sleep in healthy young volunteers. Clin Drug Investig 23:395–404
Ellsworth P, Kirshenbaum E (2010) Update on the pharmacologic management of overactive bladder: the present and the future. Urol Nurs 30:29–38
Geyer J, Gavrilova O, Petzinger E (2009) The role of p-glycoprotein in limiting brain penetration of the peripherally acting anticholinergic overactive bladder drug trospium chloride. Drug Metab Dispos 37:1371–1374
Geyer J, Gavrilova O, Schwantes U (2010) Differences in the brain penetration of the anticholinergic drugs trospium chloride and oxybutinin. UroToday Int. doi:10.3834/uij.1944-5784.2010.02.12, Volume 3, issue 1
Gillespie and Muir (1970) Species and tissue variation in extraneuronal and neuronal accumulation of noradrenaline. J Physiol 206:591–604
Gish P, Mosholder AD, Truffa M, Johann-Liang R (2009) Spectrum of central anticholinergic adverse effects associated with oxybutynin: comparison of pediatric and adult cases. J Pediatr 155:432–434
Gründemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H (1994) Drug excretion mediated by a new prototype of polyspecific transporter. Nature 372:549–552
Gründemann D, Schechinger B, Rappold GA, Schömig E (1998) Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci 1:349–351
Haenisch B, Bönisch H (2010) Interaction of the human plasma membrane monoamine transporter (hPMAT) with antidepressants and antipsychotics. Naunyn Schmiedebergs arch pharmacol 381:33–39
Hayer-Zillgen M, Bruss M, Bönisch H (2002) Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol 136:829–836
Hegde SS (2009) Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol 147:S80–S87
Kay G, Malhotra B, Michel MC (2009) Assessment of central nervous system access of a new antimuscarinic drug, fesoterodine. J Urol 181(Suppl):84
Kindla J, Fromm MF, König J (2009) In vitro evidence for the role of OATP and OCT uptake transporters in drug-drug interactions. Expert Opin Drug Metab Toxicol 5:489–500
Kitamura S, Maeda K, Sugiyama Y (2008) Recent progresses in the experimental methods and evaulation strategies of transporter functions for the prediction of the pharmacokinetics in humans. Naunyn Schmiedebergs Arch Pharmacol 377:617–628
Koepsell H, Endou H (2004) The SLC22 drug transporter family. Pflugers Arch 447:666–676
Koepsell H, Lips K, Volk C (2007) Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24:1227–1251
Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W, Wessler I, Schwantes U, Koepsell H, Kummer W (2007) Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol 51:1042–1053
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Michel MC, Schafers RF, de la Rosette JJ (2009) Drug-drug interactions in urology. Urologe A 48:264–269
Neuhoff S, Ungell AL, Zamora I, Artursson P (2003) pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug-drug interactions. Pharm Res 20:1141–1148
Schömig E, Lazar A, Gründemann D (2006) Extraneuronal monoamine transporter and organic cation transporters 1 and 2: a review of transport efficiency. Handb Exp Pharmacol 175:151–180
Singh-Franco D, Machado C, Tuteja S, Zapantis A (2005) Trospium chloride for the treatment of overactive bladder with urge incontinence. Clin Therapeut 27:511–530
Staskin D, Kay G, Tannenbaum C, Goldman HB, Bhashi K, Ling J, Oefelein MG (2010) Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract. doi:10.1111/j.1742-1241.2010.02433.x
Staskin DR, Harnett MD (2004) Effect of trospium chloride on somnolence and sleepiness in patients with overactive bladder. Curr Urol Rep 5:423–426
Thiebaut F, Tsuruo F, Hamada H, Gottesman MM, Pastan I, Willingham MC (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissue. Proc Natl Acad Sci USA 84:7724–7738
Todorova A, Vonderheid-Guth B, Dimpfel W (2001) Effects of tolterodine, trospium chloride, and oxybutynin on the central nervous system. J Clin Pharmacol 41:636–644
Wenge B, Bönisch H (2008) N-Ethylmaleimide differentially inhibits substrate uptake by and ligand binding to the noradrenaline transporter. Naunyn Schmiedebergs Arch Pharmacol 377:255–265
Wenge B, Bönisch H (2009) Interference of the noradrenergic neurotoxin DSP4 with neuronal and nonneuronal monoamine transporters. Naunyn Schmiedebergs Arch Pharmacol 380:523–529
Wiedemann A, Schwantes PA (2007) Antimuscarinic drugs for the treatment of overactive bladder: are they really all the same?—A comparative review of data pertaining to pharmacological and physiological aspects. Eur J Ger 9:S29–S42
Yamaguchi O (2010) Antimuscarinics and overactive bladder: other mechanism of action. Neurourol Urodyn 29:112–115
Zolk O, Solbach TF, König J, Fromm MF (2009) Structural determinants of inhibitor interaction with the human cation transporter OCT2 (SLC22A2). Naunyn Schmiedebergs arch pharmacol 379:337–348
Acknowledgement
The study was supported by Dr. R. Pfleger GmbH (Bamberg, Germany) by organising and financing the labelling of both drugs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wenge, B., Geyer, J. & Bönisch, H. Oxybutynin and trospium are substrates of the human organic cation transporters. Naunyn-Schmied Arch Pharmacol 383, 203–208 (2011). https://doi.org/10.1007/s00210-010-0590-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-010-0590-x