Abstract
The effects of a lectin (AaL) from seeds of Araucaria angustifolia were investigated in the model of rat paw edema. In vivo anti-and pro-inflammatory activities, role of sugar residues, inflammatory mediators and systemic toxicity were assessed. Intravenous injection of AaL (0.1–1 mg/kg) dose-dependently inhibited the dextran-induced increase in edema and vascular permeability, which were prevented by association of the lectin with its binding sugar N-acetyl-glucosamine (Glyc-Nac). AaL also significantly inhibited edema induced by serotonin (18%) and compound 48/80 (33%), but not edema induced by histamine. In contrast, when applied by the s.c. route, AaL evoked a paw edema that peaked 1 h later and was partially prevented by association with Glyc-Nac (59%) or by prior i.v. administration of the lectin itself (38.8%). This AaL edematogenic activity was significantly inhibited by pentoxifylline (44.4%) or dexamethasone (51%) and also by depletion of rat paw mast cells (45.6%), but not byl-N-nitro-arginine methyl ester or indomethacin, excluding involvement of nitric oxide and prostaglandins. Treatment of animals with a single anti-inflammatory dose of AaL (1 mg/kg, i.v.) for 7 days did not affect rat corporal mass, liver, kidney, spleen or stomach wet weight, blood leukocyte count, and urea, creatinine or serum transaminase activity. Systemic toxicity was apparent only at much higher doses (LD50=88.3 mg/kg) than those required for the anti-inflammatory effect. Summarizing, AaL exerts anti-and pro-edematogenic actions via interaction with its specific lectin domain. These actions may share a common pathway involving either activation or inhibition of inflammatory mediators from resident mast cells.






Similar content being viewed by others
References
Alencar NMN, Teixeira EH, Assreuy AMS, Cavada BS, Flores CA, Ribeiro RA (1999) Leguminous lectins as tools for studying the role of sugar residues in leukocyte recruitment. Mediat Inflamm 8:107–113
Alencar NMN, Assreuy AMS, Alencar VBM, Melo SC, Ramos MV, Cavada BS, Cunha FQ, Ribeiro RA (2003) The galactose-binding lectin from Vatairea macrocarpa seeds induces in vivo neutrophil migration by indirect mechanism. Int J Biochem Cell B 35:1674–1681
Alencar NMN, Assreuy AMS, Criddle DN, Souza EP, Soares PM., Havt A, Aragão KS, Bezerra DP, Ribeiro RA, Cavada BS (2004) Vatairea macrocarpa lectin induces paw edema with leukocyte infiltration. Protein Peptide Lett 11:195–200
Alencar NMN, Cavalcante CF, Vasconcelos MP, Leite KB, Assreuy AMS, Cavada BS, Nogueira NAP, Vale MR (2005) Antiinflammatory and antimicrobial effect of lectin from Lonchocarpus sericeus seeds in an experimental model of infectious peritonitis. J Pharm Pharmacol 57:912–922
Assreuy AMS, Shibuya MD, Martins GJ, Souza MLP, Cavada BS, Moreira RA, Oliveira JTA, Ribeiro RA, Flores CA (1997) Anti-inflammatory effect of glucose-mannose binding lectins isolated from Brazilian beans. Mediat Inflamm 6:201–210
Assreuy AMS, Martins GJ, Moreira ME, Brito GA, Cavada BS, Ribeiro RA, Flores CA (1999) Prevention of cyclophosphamide-induced hemorrhagic cystitis by glucose-mannose binding plant lectins. J Urol 161:1988–1993
Bach MK, Brashler JR (1975) Inhibiton of IgE and compound 48/80-induced histamine release by lectins. Immunology 29:371–386
Bernot D, Peiretti F, Canault M, Juhan-Vague I, Nalbone G (2005) Upregulation of TNF-alpha-induced ICAM-1 surface expression by adenylate cyclase-dependent pathway in human endothelial cells. J Cell Physiol 202:434–441
Berstad J (1980) Dextran-induced release of serotonin from isolated rat peritoneal mast cells. Acta Pharmacol Toxicol 47:213–216
Castex N, Fioramonti J, Fargeas MJ, More J, Bueno L (1994) Role of 5-HT3 receptors and afferent fibers in the effects of mast cell degranulation on colonic motility in rats. Gastroenterology 107:976–984
Cirino G, Peers SH, Flower RJ, Browning JL, Pepinsky RB (1989) Human recombinant lipocortin 1 has acute local anti-inflammatory properties in the rat paw edema test. Proc Natl Acad Sci 86:3428–3432
Coelho AM, Fioramonti J, Bueno L (1998) Mast cell degranulation induces delayed rectal allodynia in rats: role of histamine and 5-HT. Digest. Dis. Sci. 43:727–737
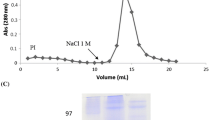
Datta PK, Figueroa MOC, Lajolo FM (1991) Purification and characterization of two major lectins from Araucaria brasiliensis syn. Araucaria angustifolia seeds (pinhão). Plant Physiol 97:856–862
Dey RD and Hoffpauir J (1984) Ultrastructural immunocytochemical localization of 5-hydroxytryptamine in gastric enterochromaffin cells. J Histochem Cytochem 32:661–666
Dinarello CA (2000) Interleukin-18, a proinflammatory cytokine. Eur Cytokine Network 11:483–486
Dinarello CA (2002) The IL-1 family and inflammatory diseases. Clin Exp Rheumatol 20:1–13
Di Rosa M, Giroud JP, Willoughby DA (1971) Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 104:15–29
Feitosa RF, Melciades GB, Assreuy AMS, Rocha MF, Ribeiro RA, Lima AA (2002) The pharmacological profile of ovoalbumin-induced paw oedema in rats. Mediat Inflamm 11:155–163
Gomes JC, Ferreira RR, Cavada BS, Moreira RA, Oliveira JT (1994) Histamine release induced by glucose (mannose)-specific lectins isolated from Brazilian beans. Comparison with concanavalin A. Agents Actions 41:132–135
Jaffuel D, Demoly P, Gougat C, Mautino G, Bousquet J, Mathieu M (1999) Rifampicin is not an activator of the glucocorticoid receptor in A549 human alveolar cells. Mol Pharmacol 55:841–846
Kankuri E, Vaali K, Korpela R, Pakari I, Vapatalo H, Moilanen E (2001) Effects of a COX-2 preferential agent nimesulide on TNBS-induced acute inflammation in the gut. Inflammation 25:301–310
Kim TW, Moon HB, Kim SJ (2003) Interleukin-10 is up-regulated by prolactin and serum-starvation in cultured mammary epithelial cells. Mol Cells 31:168–172
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Landucci ECT, Antunes E, Donato JL, Faro R, Hyslop S, Marangoni S, Oliveira B, Cirino G, De Nucci G (1995) Inhibition of carrageenan-induced rat paw edema by crotapotin, a polypeptide complexed with phospholipase A2. Brit J Pharmacol 114:578–583
Leme JG, Wilhelm DL (1975) The effects of adrenalectomy and corticosterone on vascular permeability responses in the skin of the rat. Brit J Exp Pathol 56:402–407
Lo TN, Almeida AP, Beaven MA (1982) Dextran and carrageenan evoke different inflammatory responses in rat with respect to composition of infiltrates and effect of indomethacin. J Pharmacol Exp Ther 221:261–267
Lyons CR (1995) The role of nitric oxide in inflammation. Adv Immunol 60:323–371
Marone G, Genovese A, Granata F, Forte V, Detoraki APA, Triggiani M (2002) Pharmacological modulation of human mast cells and basophils. Clin Exp Allergy 32:1682–1689
Matsuda K, Niitsuma A, Uchida MK, Suzuki-Nishimura T (1994) Inhibitory effects of sialic acid-or N-acetylglucosamine-specific lectins on histamine release induced by compound 48/80, bradykinin and a polyethylenimine in rat peritoneal mast cells. Jpn J Pharmacol 64:1–8
Mekori YA, Metcalfe DD (2000) Mast cells in innate immunity. Immunol Rev 173:131–140
Metcalfe DD, Baram D, Mekori YA (1997) Mast cells. Physiol Rev 77:1033–1079
Michel MC, König G, Mohr K, Simmet T (2005) Editorial guidelines for manuscripts on the pharmacology of plant extracts. Naunyn-Schmiedeberg’s Arch Pharmacol. 371:349–350
Miller LLC, Tainter TL (1944) Stimation of the DL50 and its error by means of logarithmin-probits graphpaper. Proc Soc Exp Biol Med 57:261–268
Moncada S, Palmer RMJ, Higgs EA (1991) Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 43:109–142
Moodley I, Mongan JL, Foreman JC (1982) Histamine release induced by dextran: the nature of the dextran receptor. Eur J Pharmacol 83:69–81
Mossner R, Lesch KP (1998) Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immun 12:249–271
Nagata K, Fujimiva M, Sugiura H, Uehara M (2001) Intracellular localization of serotonin in mast cells of the colon in normal and colitis rats. Histochem J 33:559–568
Ohta Y, Kobayashi T, Inui K, Yoshino J, Nakasazawa S (2003) Protective effect of teprenone against acute gastric mucosal lesions induced by compound 48/80, a mast cell degranulator, in rats. J Pharma Sci 93:337–346
Peumans WJ and Van Damme EJM (1995) Lectins as plant defense proteins. Plant Physiol 109:347–352
Santucci L, Fiorucci S, Giansanti M, Brunori PM, Di Matteo FM, Morelli A (1994) Pentoxifylline prevents indomethacin induced acute gastric mucosal damage in rats: role of tumour necrosis factor alpha. Gut 35:909–915
Sharon N, Lis H (1990) Legume lectins-a large family of homologous proteins. FASEB J 4:3198–3208
Souza GEP, Ferreira SH (1985) Blockade by antimacrophage serum of the migration of PMN neutrophils into the inflammed peritoneal cavity. Agents Actions 17:97–103
Acknowledgements
M.R.L.M. (graduate student) and R.C. (undergraduate student) are recipients of fellowships from Fundação Cearense de Amparo à Pesquisa (FUNCAP) and CNPq, respectively. B.S.C. and A.M.S.A. are senior investigators of CNPq (Brazil).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mota, M.R.L., Criddle, D.N., Alencar, N.M.N. et al. Modulation of acute inflammation by a chitin-binding lectin from Araucaria angustifolia seeds via mast cells. Naunyn-Schmied Arch Pharmacol 374, 1–10 (2006). https://doi.org/10.1007/s00210-006-0097-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-006-0097-7