Abstract
The epithelial Ca2+ channels TRPV5 and TRPV6 represent a new family of Ca2+ channels that belongs to the superfamily of transient receptor potential channels. TRPV5 and TRPV6 constitute the apical Ca2+ entry mechanism in active Ca2+ transport in kidney and intestine. The central role of TRPV5 and TRPV6 in active Ca2+ (re)absorption makes it a prime target for regulation to maintain Ca2+ balance. This review covers the hormonal regulation, interaction with accessory proteins and (patho)physiological implications of these epithelial Ca2+ channels.
Similar content being viewed by others
Introduction
The maintenance of the body Ca2+ balance is of crucial importance for many vital physiological functions including neuronal excitability, muscle contraction and bone formation. The extracellular Ca2+ concentration is tightly controlled by the concerted action of intestinal Ca2+ absorption, exchange of Ca2+ from bone and renal Ca2+ reabsorption (Fukugawa and Kurokawa 2002; Hurwitz 1996). Both in kidney and intestine, Ca2+ can (re)enter the extracellular fluid by passive paracellular as well as active transcellular Ca2+ transport (Bindels 1993; Wasserman and Fullmer 1995). Active Ca2+ (re)absorption is the primary target for regulation by calciotropic hormones, including 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) and parathyroid hormone (PTH), enabling the organism to regulate Ca2+ (re)absorption and respond to the body’s demand (Fig. 1; Hoenderop et al. 2000b; Wasserman and Fullmer 1995). Active absorption of dietary Ca2+ occurs primarily in the proximal small intestine, while in kidney active Ca2+ reabsorption is restricted to the distal convoluted tubule (DCT) and the connecting tubule (CNT; Bronner et al. 1986; Friedman and Gesek 1995).
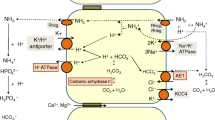
Mechanism of active epithelial Ca2+ transport. Active transcellular Ca2+ transport is generally regarded as the site for fine-tuning Ca2+ (re)absorption and is regulated by calciotropic hormones. The active form of vitamin D, 1,25 (OH)2D3, stimulates the individual steps of transcellular Ca2+ transport by increasing the expression levels of the luminal Ca2+ channels, calbindins, and the extrusion systems. Active and transcellular Ca2+ transport is carried out as a three-step process. Following entry of Ca2+ through the (hetero)tetrameric epithelial Ca2+ channels, TRPV5 and TRPV6, Ca2+ bound to calbindin diffuses to the basolateral membrane. At the basolateral membrane, Ca2+ is extruded via an ATP-dependent Ca2+-ATPase (PMCA1b) and a Na+-Ca2+-exchanger (NCX1). In this way, there is net Ca2+ (re)absorption from the luminal space to the extracellular compartment.
At the cellular level, active Ca2+ (re)absorption is generally envisaged as a three-step process (Fig. 1), consisting of passive entry of Ca2+ across the luminal or apical membrane, cytosolic diffusion of Ca2+ bound to vitamin D3-sensitive Ca2+-binding proteins (calbindin-D28K and/or calbindin-D9K) and active extrusion of Ca2+ across the opposite basolateral membrane by the Na+-Ca2+-exchanger (NCX1) and/or Ca2+-ATPase (PMCA1b; Bronner 2003; Hoenderop et al. 2002b). The molecular identity of the apical Ca2+ entry pathway remained elusive until the epithelial Ca2+ channels TRPV5 (previously named ECaC1; Hoenderop et al. 1999) and TRPV6 (previously named Ca2+ transporter 1) were identified (Montell et al. 2002b; Peng et al. 1999). These channels convey the rate-limiting step in active Ca2+ transport and play, therefore, a pivotal role in Ca2+ homeostasis. This review highlights the regulation and (patho)physiological implications of these two epithelial Ca2+ channels.
Molecular features of TRPV5 and TRPV6
The genes of TRPV5 and TRPV6 comprise 15 exons, encoding proteins of about 730 amino acids, sharing 75% homology (Hoenderop et al. 1999; Muller et al. 2000b; Peng et al. 1999, 2001a). TRPV5 and TRPV6 constitute a distinct class of highly Ca2+-selective channels within the transient receptor potential (TRP) superfamily, which encompasses a diversity of non-voltage operated cation channels (Clapham et al. 2001; Harteneck et al. 2000; Montell et al. 2002b). These two channels belong to the TRPV (vanilloid) subfamily, which is one of the subfamilies comprising this superfamily (Hoenderop et al. 2005; Montell et al. 2002a; Peng et al. 2003; Vennekens et al. 2002).
The tissue distribution of TRPV5 and TRPV6 has been studied extensively by Northern blot, RT-PCR analysis and immunohistochemistry. In human, both channels are co-expressed in organs that mediate transcellular Ca2+ transport such as duodenum, jejunum, colon, and kidney, but also in pancreas, prostate, mammary, sweat and salivary gland (Hirnet et al. 2003; Hoenderop et al. 1999, 2000a, 2000b; Janssen et al. 2002; Muller et al. 2000a; Peng et al. 1999, 2000, 2001a; Weber et al. 2001; Wissenbach et al. 2001; Zhuang et al. 2002). In general, TRPV5 seems to be the major isoform in kidney, whereas TRPV6 is more ubiquitously expressed with the highest concentrations in the prostate, stomach, brain, lung and small intestine. The relative mRNA level of TRPV5 and TRPV6 in tissues co-expressing these channels is different. For instance, in duodenum the TRPV5 mRNA levels are much lower than that of TRPV6 or even below detection levels (Song et al. 2003; van Abel et al. 2003; van Cromphaut et al. 2001). In kidney, however, TRPV5 mRNA is at least 100 times higher expressed compared to TRPV6. Variations in TRPV5 and TRPV6 mRNA expression patterns found in several studies could have resulted from differences between species or the employed detection methods. Ultimately, it will be important to analyze the expression differences quantitatively at the protein level.
The structure of TRPV5 and TRPV6 shows typical topology features shared by all members of the TRP family, including six transmembrane regions, a short hydrophobic stretch between transmembrane segments 5 and 6, which is predicted to form the Ca2+ pore, and large intracellular N- and C-terminal domains (Hoenderop et al. 2002b). These intracellular regions contain several conserved putative regulatory sites that might be involved in regulation of channel activity and trafficking, like phosphorylation sites, PDZ motifs (post synaptic density protein, disk large, zona occludens), and ankyrin repeat domains (Hoenderop et al. 2002b).
Electrophysiological studies using human embryonic kidney cells (HEK293), heterologously expressing TRPV5 or TRPV6, show that both channels are permeable for monovalent and divalent cations with a high selectivity for Ca2+ (Gunthorpe et al. 2002; Hoenderop et al. 2001b; Vennekens et al. 2002). The characteristic pore region of TRPV5 and TRPV6 is unique for its high Ca2+ selectivity, in which a single aspartic residue at position number 542 (D542) is crucial for Ca2+ permeation (Nilius et al. 2001b; Vennekens et al. 2001). The current-voltage relationship of TRPV5 and TRPV6 shows inward rectification and they exhibit a Ca2+-dependent feedback mechanism regulating channel activity (Nilius et al. 2001a; Vennekens et al. 2000). However, TRPV5 and TRPV6 exhibit different channel kinetics with respect to Ca2+-dependent inactivation and recovery, Ba2+ selectivity and sensitivity for inhibition by ruthenium red (Hoenderop et al. 2001b; Nilius et al. 2002; Vennekens et al. 2000). Furthermore, it has been demonstrated that TRPV5 and TRPV6 can operate as homo- and heterotetrameric ion channels, which implies that four of the aspartic residues (D542) form a negatively charged ring operating as a selectivity filter for Ca2+ (Hoenderop et al. 2003b).
Regulation of TRPV5 and TRPV6
A tight control of the amount of Ca2+ influx across the plasma membrane is important in many physiological processes. A delicate regulation of the expression and activity of TRPV5 and TRPV6 is, therefore, of utmost importance to maintain the extracellular Ca2+ balance.
Transcriptional regulation
Vitamin D
The active metabolite of vitamin D, 1,25(OH)2D3, is an important regulator of the body Ca2+ homeostasis, having a significant role in the regulation of Ca2+ (re)absorption (Reichel et al. 1989). This is reflected by mutations in the genes encoding 25-hydroxyvitamin D3-1α-hydroxylase (1α-OHase), a renal enzyme controlling its synthesis, and the 1,25(OH)2D3-receptor (VDR; Hughes et al. 1988; Kitanaka et al. 1998). As TRPV5 and TRPV6 form the rate-limiting entry step in active Ca2+ (re)absorption, regulation of these channels by 1,25(OH)2D3 has been investigated using different vitamin D-deficiency models. The first evidence for a vitamin D-sensitivity was obtained from in vivo studies in which rats were depleted for vitamin D3. The reduced renal TRPV5 expression and accompanied hypocalcemia were completely restored after 1,25(OH)2D3 supplementation (Hoenderop et al. 2001a). Similar results were obtained using different animal models and cell lines, including VDR and 1α-OHase knockout mice and the human intestinal cell line Caco-2, confirming the vitamin D-dependent regulation of TRPV5 and TRPV6 (Fleet et al. 2002; Hoenderop et al. 2002a; van Abel et al. 2003; van Cromphaut et al. 2001; Weber et al. 2001; Wood et al. 2001). Together these findings indicate that the expression of TRPV5 and TRPV6 is indeed controlled by 1,25(OH)2D3.
Dietary Ca2+
Several aforementioned studies provide evidence that the TRPV5 and TRPV6 channels are regulated by 1,25(OH)2D3, however, it is difficult to distinguish the effects of hypocalcemia from those of vitamin D-deficiency. Interestingly, dietary Ca2+ enrichment resulted in the normalization of the reduced renal TRPV5 and duodenal TRPV6 expression levels in 1α-OHase knockout mice, as well as for the other Ca2+ transport proteins participating in active Ca2+ (re)absorption (Hoenderop et al. 2002a; van Abel et al. 2003). In mice lacking a functional vitamin D receptor, dietary Ca2+ levels regulated the expression of TRPV5 and TRPV6 in kidney and duodenum, respectively (Panda et al. 2004; van Cromphaut et al. 2001; Weber et al. 2001). These findings indicate that dietary Ca2+ can exert a regulatory effect on TRPV5 and TRPV6 expression independent of 1,25(OH)2D3.
Estrogen
It is known that estrogen is involved in Ca2+ homeostasis and that the deleterious effects of estrogen deficiency after menopause result in a negative Ca2+ balance associated with postmenopausal osteoporosis (Prince 1994; Young and Nordin 1969). In addition, it has been demonstrated that estrogen deficiency after menopause is associated with increased renal Ca2+ loss and intestinal Ca2+ malabsorption, which can be corrected by estrogen replacement therapy (Colin et al. 1999; Gennari et al. 1990; Nordin et al. 1991; Prince et al. 1991). Therefore, the effect of estrogen on the proteins involved in active Ca2+ (re)absorption was investigated. In ovariectomized 1α-OHase knockout mice 17β-estradiol replacement therapy resulted in upregulation of renal TRPV5 mRNA and protein levels, which was accompanied by a normalization of serum Ca2+ levels. In addition, in duodenum both TRPV5 and TRPV6 mRNA levels were upregulated in these supplemented mice (van Abel et al. 2002, 2003). Thus, TRPV5 and TRPV6 expression is transcriptionally controlled by estrogen in a vitamin D-independent manner. These data suggest that the function of estrogen in the maintenance of Ca2+ balance might be at least in part fulfilled by controlling (re)absorption of the amount of Ca2+ through the regulation of TRPV5 and TRPV6.
Regulation of TRPV5 and TRPV6 membrane expression
A first indication for trafficking of TRPV5 towards the plasma membrane came from immunohistochemical studies of kidney sections. The late part of the DCT (DCT2) mainly express TRPV5 along the apical domain, whereas the CNT display a more cytoplasmic TRPV5 staining (Loffing et al. 2001). This suggests that TRPV5 is present in intracellular compartments from where it can be shuttled to the plasma membrane in a controlled fashion. Trafficking of TRPV5 or TRPV6 towards the plasma membrane provides a short-term regulatory mechanism to increase renal and intestinal Ca2+ uptake, respectively.
S100A10-annexin 2 complex
The S100A10-annexin 2 complex plays an important role in biological processes including endocytosis, exocytosis and membrane-cytoskeletal interactions (Gerke and Moss 2002). Recently, we demonstrated a regulatory role for the S100A10-annexin 2 complex in the trafficking of TRPV5 and TRPV6 (van de Graaf et al. 2003). S100A10 and annexin 2 were present along the apical membrane of TRPV5-expressing tubules and along the brush-border membrane of duodenum, which is in agreement with the TRPV6 localization. Moreover, disruption of the S100A10-binding motif in TRPV5 or TRPV6 (Table 1) prevented the facilitation of Ca2+ inward currents, which was accompanied by a major disturbance in their subcellular localization (van de Graaf et al. 2003). Importantly, downregulation of annexin 2 using annexin 2-specific siRNAs significantly inhibited the currents through TRPV5, indicating that annexin 2 in conjunction with S100A10 is crucial for TRPV5 activity (van de Graaf et al. 2003). These results clearly show that the S100A10-annexin 2 complex is a significant component for the trafficking of the epithelial Ca2+ channels towards the plasma membrane and, therefore, the functionality of these channels.
PDZ motifs
PDZ motifs are recognized by PDZ domains that are modular protein interaction domains that play a role in protein targeting and protein complex assembly (Hung and Sheng 2002). TRPV5 and TRPV6 contain a PDZ motif, which could bind to a PDZ domain-harboring protein. The Na+-H+- exchanger regulating factors, NHERF1 or NHERF2, have been shown to modulate the targeting and trafficking of several renal proteins, like the epithelial Na+-H+- exchanger NHE3 and epithelial K+ channel ROMK1, as well as members of the TRP superfamily (Mery et al. 2002; Shenolikar and Weinman 2001; Yun et al. 2002). Recently, it has been demonstrated that TRPV5 forms a complex, involving NHERF2 (Table 1) and serine/threonine kinases SGK1 and 3. The concerted action of these proteins increased cell surface TRPV5 abundance and activity (Embark et al. 2004).
Ankyrin repeats
In general, ankyrins link transporters and cell adhesion molecules to the spectrin-based cytoskeletal elements in specialized membrane domains (Bennett and Lambert 1999). Ankyrin-binding proteins include the voltage-dependent Na+ channel, Na+-K+-ATPase, Na+-Ca2+- exchanger, IP3 receptor and ryanodine receptor Ca2+ release channels. The membrane-binding domain of ankyrins is comprised of one or more copies of a 33-residue repeat known as the ankyrin repeat. This protein-protein interaction module is involved in a diverse set of cellular functions, and consequently, defects in ankyrin repeat proteins result in a number of human diseases (Mosavi et al. 2002, 2004). TRPV5 and TRPV6 contain several ankyrin repeat domains in their amino-terminal region, which could be involved in the maintenance and targeting of these channels to specific membrane regions, as has been demonstrated for a neural-specific isoform of ankyrin in the localization of Na+ channels (Bennett et al. 1999). In addition, recent studies on TRPV6 showed that a specific N-terminal domain encompassing the third ankyrin repeat is required for the assembly of TRPV6 subunits within a functional tetramer (Table 1; Erler et al. 2004). This repeat initiates a molecular zippering process that proceeds past the fifth ankyrin repeat and creates an intracellular anchor that is necessary for functional subunit assembly. Moreover, deletion of this repeat or mutation of critical residues within this repeat rendered non-functional channels and prevented TRPV6-TRPV6 association (Erler et al. 2004). In addition, experiments with TRPV5 showed that predominantly the N-tail and to a lesser extent the C-tail are critical for channel assembly (Table 1). The assembly domain in the N-tail of TRPV5 overlapped with the first ankyrin repeat. Deletion of these assembly domains abolished channel assembly and thereby the trafficking towards the plasma membrane and subsequent channel activity (Chang et al. 2004). However, Erler et al. indicated that tetramer formation is not essential for trafficking of TRPV6 to the cell surface since deletion of the N-terminal assembly domain did not affect plasma membrane staining (Erler et al. 2004). Deleting or mutating assembly domains could cause a change in tertiary structure and/or prevent the interaction with auxiliary proteins, thereby affecting channel trafficking and activity. For instance, the assembly domain in the C-terminal tail of TRPV5, the MLERK sequence, is also known to bind the protein 80K-H (Gkika et al. 2004). Impaired trafficking observed in the MLERK-mutant of TRPV5 could be explained by the inability to interact with the necessary auxiliary proteins. However, 80K-H was identified as a Ca2+ sensor regulating TRPV5 activity at the plasma membrane rather than a role in routing towards the plasma membrane as discussed below. Importantly, these studies provide evidence that ankyrin repeats in the N-tail of TRPV5 and TRPV6 are essential for subunit assembly (Chang et al. 2004; Erler et al. 2004). In addition, it is likely that assembly also occurs in the N-tail and C-tail of TRPV5 and TRPV6, because they share more than 75% homology at the amino acid level, raising the possibility that both N-tail and C-tail assembled together in order to form functional heterotetrameric channel complexes of TRPV5 and TRPV6 (Hoenderop et al. 2003b). Detailed sequence comparison of the N- and C-tails of the TRPV5 and TRPV6 channels reveals significant differences, which may account for the unique electrophysiological properties (Hoenderop et al. 2001b). In addition, the assembly domains could differ between the two channels in order to specifically allow self-assembly or the formation of heterotetrameric channels.
Regulation of TRPV5 and TRPV6 activity
TRPV5 and TRPV6 are constitutively active, unlike many other TRP channels that are activated by binding of ligands (Montell et al. 2002b). This implies that in order to regulate TRPV5/6 activity, short-term acting mechanisms must exist that control the activity of these channels located on the plasma membrane.
Ca2+-dependent inactivation
Intracellular Ca2+ exerts a negative feedback mechanism on TRPV5 and TRPV6 activity (Hoenderop et al. 2001b). This Ca2+-dependent inactivation is different between TRPV5 and TRPV6. The inactivation of TRPV6 is characterized by a slow decline after an initial fast inactivation phase, whereas TRPV5 only shows the slow inactivation phase (Nilius et al. 2001a, 2002). In line with a Ca2+-dependent regulation is the observation that chelation of intracellular Ca2+ with ethylene glycol-bis(b-aminoethyl ether)-N,N,N′,N′-tetra acetic acid (EGTA) or 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acethoxymethylester (BAPTA) resulted in increased channel activity (Bodding and Flockerzi 2004; Hoenderop et al. 2001b; Peng et al. 1999). Together, these data indicate that intracellular Ca2+ exerts a negative feedback mechanism on TRPV5 and TRPV6 activity. In addition, it has been demonstrated that the difference in Ca2+-dependent inactivation between TRPV5 and TRPV6 is restricted to the first intracellular loop between transmembrane domain 2 and 3 (Nilius et al. 2002).
Calmodulin
Calmodulin (CaM) plays a pivotal role in Ca2+-dependent inactivation, acting as a Ca2+ sensor, thereby facilitating both activation and inactivation of ion channels, including voltage- and ligand-gated Ca2+ channels (Saimi and Kung 2002). Moreover, CaM has been shown to modulate several channels from the TRP superfamily (Boulay 2002; Numazaki et al. 2003; Phillips et al. 1992; Singh et al. 2002; Tang et al. 2001; Trost et al. 1999, 2001; Warr and Kelly 1996; Zhang et al. 2001). TRPV5 and TRPV6 activity is negatively regulated by the intracellular Ca2+ concentration and CaM could mediate the regulation of the activity of both channels. Niemeyer et al. showed for the first time that CaM binds to the C-terminus of human TRPV6 in a Ca2+-dependent manner (Table 1; Niemeyer et al. 2001). In addition, Nilius et al. indicated that the rabbit TRPV5 C-terminal region is also important for the Ca2+-dependent inactivation process (Nilius et al. 2003). Recently, CaM was shown to bind to the C- and N-tails of TRPV5 and TRPV6 as well as the transmembrane domain of TRPV6 in a Ca2+-dependent fashion (Table 1). Furthermore, electrophysiological measurements of HEK293 cells heterologously co-expressing Ca2+-insensitive CaM mutants and TRPV6 revealed a significantly reduced inward Ca2+ current, whereas, no effect was demonstrated on currents of TRPV5-expressing cells (Lambers et al. 2004). It remains, therefore, to be established whether CaM is the general Ca2+ sensor or that other processes play a role in the Ca2+-dependent inactivation of TRPV5 (Hoenderop et al. 2001b; Lambers et al. 2004; Nilius et al. 2003). Moreover, the high affinity EF-hand Ca2+-binding sites of CaM were demonstrated to contribute primarily to the observed CaM effect upon TRPV6 activity (Lambers et al. 2004). The C-terminal CaM-binding site of TRPV6 is identical between mouse and rat, but not similarly present in human TRPV6, which might explain the previous observation that CaM binding to human TRPV6 is localized to a different region (Hirnet et al. 2003; Lambers et al. 2004; Niemeyer et al. 2001). Together, these results suggest that CaM positively affects TRPV6 activity.
80K-H
80K-H was initially identified as a PKC substrate and subsequently associated with intracellular signaling (Hirai and Shimizu 1990). A study by Gkika et al. demonstrated a functional role for 80K-H in the Ca2+-dependent regulation of TRPV5 channel activity (Gkika et al. 2004). TRPV5 and 80K-H co-localized in 1,25 (OH)2D3-responsive epithelia, forming a heteromeric complex along the plasma membrane. Furthermore, 80K-H acted like a Ca2+-sensor, facilitating the Ca2+ influx through TRPV5 after binding Ca2+ to its two EF-hand structures. Inactivation of the EF-hand pair reduced the TRPV5-mediated Ca2+ current and increased TRPV5 sensitivity to intracellular Ca2+, accelerating the feedback inhibition of channel activity. The membrane localization of 80K-H and TRPV5 was not altered, suggesting that 80K-H has a direct effect on TRPV5 activity (Gkika et al. 2004). The 80K-H binding-site identified in the C-terminal tail of TRPV5 corresponds to the amino acid sequence MLERK, which is the same region where TRPV5 self-assembly can occur (Table 1; Chang et al. 2004). Regulation of TRPV5 at this site could involve competition between the proteins, thereby differently regulating the channel. More interestingly, multiple regulations could take place through the four sites available in the tetrameric channel-complex.
TRPV5 and TRPV6 in disease
The role of TRPV5 and TRPV6 in diverse Ca2+-related disorders has been considered, including variation in the urinary Ca2+ excretion during treatment with pharmaceutical agents.
Vitamin D-deficiency rickets
Vitamin D-deficiency rickets type I (VDDR-I) is an autosomal recessive disease characterized by low or undetectable levels of 1,25(OH)2D3 resulting in hypocalcemia, rickets, osteomalacia, growth retardation and failure to thrive. The disease is caused by mutations in the 1α-OHase gene (Dardenne et al. 2001; Kitanaka et al. 1998). In clinical practice, patients are treated with vitamin D analogues and/or an enriched Ca2+ diet to prevent rickets, which correct the major perturbations in Ca2+ homeostasis. As previously mentioned, in 1α-OHase knockout mice, a downregulation of renal TRPV5 as well as intestinal TRPV6 expression was demonstrated, whereas supplementation with 1,25(OH)2D3 or an enriched Ca2+ diet restored expression levels of these Ca2+ transporters and serum Ca2+ concentrations (Hoenderop et al. 2002a; van Abel et al. 2003). These data suggest that decreased channel abundance contributes to defective renal and intestinal Ca2+ absorption and, thereby, the sustained hypocalcemia in VDDR-I. In addition, patients with hereditary hypocalcemic vitamin D-deficiency rickets type II (VDDR-II), an autosomal recessive disorder caused by defects in the gene encoding the nuclear vitamin D receptor (VDR), display a similar phenotype except for the elevated serum 1,25(OH)2D3 levels. Experiments performed with VDR knockout mice revealed disturbed Ca2+ absorption and inappropriately high renal Ca2+ excretion, which was accompanied by a downregulation of TRPV5 and TRPV6 in kidney and duodenum (van Cromphaut et al. 2001; Weber et al. 2001). Together, these data demonstrate that 1,25(OH)2D3 regulates the epithelial Ca2+ channels and thus affecting Ca2+ (re)absorption. Moreover, this suggests that TRPV5 and TRPV6 play a central role in the pathophysiology of vitamin D-deficiency rickets and determine, at least in part, the clinical phenotype of VDDR-I and VDDR-II.
Idiopathic hypercalciuria and renal stone disease
Normocalcemic hypercalciuria in the absence of any known underlying cause is termed idiopathic hypercalciuria (IH). The pathogenesis of this autosomal dominant disorder is either excessive intestinal Ca2+ absorption (absorptive IH) or defective renal tubular Ca2+ reabsorption (Asplin et al. 1996). Furthermore, an increased urinary excretion of Ca2+ is a considerable risk factor and hypercalciuria is implicated in 40–50% of kidney stone formers, which represents an important clinical problem with considerable socio-economic impact (Asplin et al. 1996). Several potential candidate genes, which could be associated with idiopathic nephrolithiasis, have been analyzed using familial clustering. However, promising genes like the VDR, 1α-OHase and the Ca2+-sensing receptor (CaSR) gene were shown not to be associated with IH (Lerolle et al. 2001; Scott et al. 1998; Zerwekh et al. 1995). In addition, the TRPV5 gene was screened in nine families with a high-penetrance autosomal dominant inheritance and a phenotype suggesting a primary renal defect. However, no mutations were identified in the open reading frame containing 15 exons and 3 kb of the 5′-flanking region of the TRPV5 gene (Muller et al. 2002). Since the IH population is a heterogenous group, TRPV5 cannot be completely excluded as a candidate gene. Moreover, activating or silencing mutations in TRPV5 and TRPV6 can hypothetically lead to primary renal as well as absorptive IH. Single nucleotide polymorphisms (SNPs) in the Ca2+-sensing receptor (CaSR) have been shown to significantly increase the relative risk of hypercalciuria (Vezzoli et al. 2002). The same may be applicable to SNPs in the genes encoding the epithelial Ca2+ channels or genes involved in the regulation of TRPV5 and TRPV6 activity and, therefore, may be involved in the complex genetic background of IH.
Estrogens and postmenopausal osteoporosis
As previously mentioned, the deleterious effects of estrogen deficiency after menopause, leads to a negative Ca2+ balance associated with renal Ca2+ loss, intestinal Ca2+ malabsorption and postmenopausal osteoporosis, which can be corrected with estrogen replacement therapy (Colin et al. 1999; Nordin et al. 1991; Prince et al. 1991; Young and Nordin 1969). In addition, estrogen has been implicated in the protection against Ca2+ nephrolithiasis via an increased reabsorption of Ca2+. In premenopausal women kidney stone formation is less commonly than in age-matched men, which is associated with a lower urinary Ca2+ excretion (Heller et al. 2002; Soucie et al. 1994). As shown by van Abel et al. estrogen has a stimulatory effect on the expression of active Ca2+ transport proteins in both kidney and duodenum, including TRPV5 and TRPV6 (van Abel et al. 2002, 2003). Thus, the presence of estrogen may protect premenopausal women against Ca2+ nephrolithiasis by increasing TRPV5 expression and stimulating Ca2+ reabsorption. Conversely, decreased active Ca2+ transport through TRPV5 and TRPV6 as a result of estrogen deficiency would be in line with increased Ca2+ wasting and Ca2+ malabsorption in postmenopausal women.
PTH-related disorders
Parathyroid hormone (PTH) is an essential component of Ca2+ homeostasis. Secretion of PTH from the parathyroid glands is regulated by the parathyroid CaSR, which senses the ambient Ca2+ concentration (Brown et al. 1993). There are several disorders with disturbed Ca2+ homeostasis, characterized by hypo- or hyperparathyroidism. Primary hyperparathyroidism (PHPT) is a common endocrine disorder characterized by elevated PTH levels (Khosla et al. 1993). Others involve inactivating mutations in the CaSR gene, resulting in familial hypocalciuric hypercalcemia (FHH) or neonatal severe hyperparathyroidism (NSHPT; Pollak et al. 1993), whereas autosomal dominant hypocalcemia (ADH) is caused by activating mutations (Pollak et al. 1994). From the clinical symptoms of these PTH-related disorders, like hypo- or hypercalciuria and renal stone formation, it is clear that also renal Ca2+ handling is affected. We recently investigated the role of PTH in regulating renal Ca2+ transport proteins, including TRPV5 and TRPV6. Data gathered from parathyroidectomized rats showed that reducing serum PTH levels resulted in down-regulation of renal TRPV5 expression, which is in line with reduced Ca2+ reabsorption, contributing to the observed hypocalcemia. Supplementation with PTH in these rats restored the expression of TRPV5 and serum Ca2+ levels (van Abel et al., unpublished data). In addition, calcimimetic compounds were used to reduce serum PTH levels in mice. Calcimimetics are small organic compounds that, upon binding to the CaSR, enhance the sensitivity of the CaSR for Ca2+ in an allosteric fashion, thereby inhibiting PTH secretion by the parathyroid glands, and may provide a novel therapy for treating hyperparathyroidism (Nemeth 1996; Nemeth et al. 1998). Infusion of these calcimimetic compounds in normal mice resulted in decreased serum PTH levels, serum Ca2+ levels and renal expression of TRPV5 as well as reduced expression of TRPV6 in duodenum (van Abel et al., unpublished data). Thus, PTH could affect Ca2+ balance by regulating Ca2+ (re)absorption through the expression of Ca2+ transport proteins, including TRPV5 and TRPV6, and these epithelial Ca2+ channels could, therefore, be involved in the pathogenesis of PTH-related disorders.
Vitamin D analogues
Secondary hyperparathyroidism (SHPT), a common disorder in patients with chronic renal failure, develops in response to low serum levels of Ca2+ and active vitamin D metabolites. SHPT requires treatment to minimize the effect of elevated PTH on bone and other tissues (Bro and Olgaard 1997). Vitamin D compounds have been widely used in the treatment of this disorder. However, use of compounds such as 1,25(OH)2D3 has frequently been accompanied by the undesired side effects of hypercalcemia and hyperphosphatemia, which increase the risk of soft tissue and vascular calcification (Goodman et al. 2000). To avoid these side effects, vitamin D analogues have been developed with the aim of suppressing PTH secretion with minimal calcemic action (Slatopolsky et al. 2003). 1α-hydroxyvitamin D2 (1α(OH)D2) is a vitamin D prodrug, less calcemic than 1,25(OH)2D3 in animal studies (Bro et al. 1997), which must be metabolized to become active, resulting in altered pharmacokinetics relative to active vitamin D compounds. 1α,24-dihydroxyvitamin D2 (1,24(OH)2D2) is an active metabolite of 1α(OH)D2 with greatly reduced calcemic activity relative to 1,25(OH)2D3 (Knutson et al. 1995, 1997). The 1α-OHase knockout mice were used to study the activity of vitamin D compounds, namely 1,25(OH)2D3, 1α(OH)D2, and 1,24(OH)2D2, on serum Ca2+ and the expression of Ca2+ transport genes (Hoenderop et al. 2004). All three compounds were able to increase serum Ca2+ levels, although at different time-scales, reflecting their individual pharmacokinetics, thereby increasing serum 1,25(OH)2D3 levels. Interestingly, TRPV5 and TRPV6 mRNA levels in duodenum increased in parallel with serum levels of Ca2+. Effects of vitamin D compounds on Ca2+ regulatory genes in kidney were more diverse, of which 1,24(OH)2D2 did not up-regulate TRPV5. Taken together, the differences in calcemic effects of the various vitamin D compounds could result from their effect on Ca2+ channel proteins in target organs, through either reduced magnitude of induction or reduced duration of induction.
Prostate cancer
First evidence that TRP channels could be involved in the progression of certain types of cancer came from analysis of TRPM1, which gene expression is inversely correlated with the aggressiveness of malignant melanoma cells (Duncan et al. 1998). In addition, TRPM8 has originally been cloned from prostate cancer tissue (Tsavaler et al. 2001). Moreover, TRPV6 was shown to be strongly upregulated in prostate cancer (Peng et al. 2001b; Wissenbach et al. 2001, 2004). Also in carcinomas of other tissues, such as breast, thyroid, colon and ovary, TRPV6 expression is increased (Zhuang et al. 2002). Thus, TRPV6 might be a new marker for tumor progression. Indeed, northern blot analysis, in situ hybridization experiments and immunohistochemistry clearly demonstrated that TRPV6 is highly expressed in prostate cancer cells compared to healthy prostate epithelia (Fixemer et al. 2003; Wissenbach et al. 2001). In addition, TRPV6 transcripts are abundantly expressed in lymph node metastasis of prostate origin (Wissenbach et al. 2001). The appearance of TRPV6 correlates positively with the tumor grade and TRPV6 might, therefore, be a promising target for the development of drugs against prostate cancer.
Interestingly, prostate carcinoma is a hormone-sensitive malignancy and anti-androgens are frequently used in prostate cancer treatment. Peng et al. showed that in a human prostate adenocarcinoma cell line TRPV6 mRNA expression was decreased upon dihydrotestosterone treatment, whereas an androgen receptor antagonist significantly increased TRPV6 mRNA levels (Peng et al. 2001b). Furthermore, estrogens, also used therapeutically in prostate cancer, positively regulate TRPV6 (van Abel et al. 2003). In addition, vitamin D has received considerable interest as a possible anticancer drug, particularly in prostate cancer (Liu et al. 2002). VDR presence was demonstrated in prostate epithelial cells and vitamin D analogues were shown to inhibit the growth of primary cultures of human prostate tissue and cancer cell lines (Miller et al. 1992; Peehl et al. 1994; Zhao et al. 2000). As discussed previously, the positive regulation of TRPV6 by 1,25(OH)2D3 and several analogues has been clearly established (Fleet et al. 2002; Hoenderop et al. 2004; Weber et al. 2001; Wood et al. 2001). Apoptosis is induced by either depleting the cell or by overloading the cell with Ca2+ (Haverstick et al. 2000; Nicotera and Orrenius 1998; Rabinovitch et al. 2001). Therefore, an increased Ca2+ influx through TRPV6 could be involved in the apoptotic anti-cancer effects of vitamin D and other compounds on prostate carcinoma and TRPV6 might be a possible target in the development of novel anticancer therapy in prostate carcinoma.
Thiazide diuretics
Thiazide diuretics, widely used in hypertension therapy, have the unique characteristic of increasing renal Na+ excretion by inhibiting the apical Na+-Cl− cotransporter (NCC) in DCT, resulting in an increased salt and water loss and, thereby, decrease the extracellular volume (ECV; Monroy et al. 2000). Moreover, these drugs concomitantly increase Ca2+ reabsorption (Costanzo and Windhager 1978). This hypocalciuric effect provides therapeutic opportunities in for instance idiopathic hypercalciuria and nephrolithiasis. Furthermore, thiazides have been shown to increase bone mineral density and decrease fracture risk, spiking interest in the favorable long-term effects of these diuretics in counteracting osteoporosis (Reid et al. 2000). The decreased Ca2+ excretion during chronic thiazide administration has been explained by ECV contraction enhancing the paracellular Ca2+ reabsorption in proximal tubules as well as a direct stimulation of active Ca2+ reabsorption in the DCT (Costanzo et al. 1978; Ellison 2000; Friedman 1998; Friedman and Bushinsky 1999; Loffing et al. 2001; Nijenhuis et al. 2003).
In addition, in Gitelman’s syndrome, a recessive disorder caused by mutations in the gene encoding NCC, hypocalciuria is invariably present (Lemmink et al. 1996; Schultheis et al. 1998). Some hypotheses concerning the mechanism responsible for this hypocalciuria also center on stimulation of active Ca2+ transport (Ellison 2000). However, Nijenhuis et al. showed that chronic hydrochlorothiazides (HCTZ) treatment consistently decreased the mRNA expression and protein abundance of the transporters responsible for active Ca2+ reabsorption, including TRPV5, while prevention of ECV contraction during HCTZ treatment prohibited the development of hypocalciuria (Nijenhuis et al. 2003). These results suggest that ECV contraction is the critical determinant of the thiazide-induced hypocalciuria, excluding a stimulatory role of TRPV5. In addition, Loffing et al. showed recently in a mouse model for Gitelman’s syndrome that mutation in NCC did not lead to increased abundance of renal TRPV5 and NCX1. Moreover, in vivo micropuncture experiments indicated that the hypocalciuria is not related to increased Ca2+ transport rates in DCT and CNT. These results provided evidence for reduced glomerular filtration and stimulation of proximal reabsorption, which could be causative for the hypocalciuria in NCC-deficient mice and in patients with Gitelman’s syndrome (Loffing et al. 2004).
Tacrolimus
Immunosuppressant drugs, like tacrolimus, are prescribed in various disorders and to organ transplant recipients. Besides the immunosuppressive actions, tacrolimus also affects mineral homeostasis. Treatment with tacrolimus has been associated with increased bone turnover, negative Ca2+ balance and hypercalciuria. This hypercalciuric effect has been attributed to increased bone resorption and decreased renal Ca2+ reabsorption (Aicher et al. 1997; Reid and Ibbertson 1987; Reid 1997). Recently, it has been demonstrated that treatment with tacrolimus in rats enhanced renal Ca2+ wasting, which was accompanied by a decreased expression of the active Ca2+ transport proteins TRPV5 and calbindin-D28K (Nijenhuis et al. 2004). Thus, down-regulation of the renal Ca2+ transport proteins could contribute to the pathogenesis of tacrolimus-induced hypercalciuria.
TRPV5 knockout mice
As described in the aforementioned paragraphs, TRPV5 plays a critical role in active Ca2+ transport across the renal epithelia and, therefore, in Ca2+ homeostasis. In order to investigate the physiological function of TRPV5 in maintaining Ca2+ balance, TRPV5 knockout mice were generated (Hoenderop et al. 2003a). Mice lacking TRPV5 (TRPV5−/−) displayed a significant calciuresis compared to their wild-type littermates (TRPV5+/+) while remaining normocalcemic. In addition to the increased renal Ca2+ excretion, polyuria and an acidic urine were consistently observed in TRPV5−/− mice. Increasing the amount of urine and acidification of the urine reduce the potential risk of renal stone formation (Baumann 1998; Frick and Bushinsky 2003; Miller and Stapleton 1989). Micropuncture studies indicated that the renal Ca2+ transport defect was localized primarily to the DCT/CNT. Ablation of TRPV5 in the distal convolution was accompanied by a concomitant decrease in calbindin-D28K and NCX1 mRNA levels. Importantly, serum 1,25(OH)2D3 levels were elevated, causing an compensatory increase in intestinal TRPV6 and calbindin-D9K mRNA expression as well as Ca2+ absorption in TRPV5−/− mice, explaining the remaining normal serum Ca2+ levels. Furthermore, the TRPV5−/− mice exhibited significant disturbances in bone structure, including reduced trabecular and cortical bone thickness compared to TRPV5+/+ mice (Hoenderop et al. 2003a). Together, ablation of the TRPV5 gene seriously disturbed renal Ca2+ handling, resulting in compensatory intestinal hyperabsorption and bone abnormalities. These deficiencies in Ca2+ handling have been reported frequently in patients with idiopathic hypercalciuria, in which TRPV5 dysfunction could contribute to the pathogenesis. Finally, the increased 1,25(OH)2D3 levels and effects on bone structure indicate that alterations in TRPV5 may have implications for age-related bone disorders, including osteoporosis.
Conclusion
TRPV5 and TRPV6 comprise a unique pair of Ca2+ channels among the TRP superfamily. Distinctive physiological functions important for body Ca2+ homeostasis have now been established for these channels, but many areas remain open for further investigation. Dysregulation or dysfunction of these epithelial Ca2+ channels may contribute to disturbances in Ca2+ homeostasis and be associated with several diseases. In this respect, further examination of interacting proteins will disclose more detailed information concerning the molecular regulation of the Ca2+ channel activity. Furthermore, characterization of (tissue-specific) TRPV5 and TRPV6 knockout models should reveal the diseases that are associated with Ca2+ channel dysfunction.
References
Aicher L, Meier G, Norcross AJ, Jakubowski J, Varela MC, Cordier A, Steiner S (1997) Decrease in kidney calbindin-D 28 kDa as a possible mechanism mediating cyclosporine A- and FK-506-induced calciuria and tubular mineralization. Biochem Pharmacol 53:723–731
Asplin JR, Favus MJ, Coe FL (1996) Nephrolithiasis. In: Rector BA (ed) The kidney, 5th edn. Saunders, Philadelphia, pp 1893–1935
Baumann JM (1998) Stone prevention: why so little progress? Urol Res 26:77–81
Bennett V, Lambert S (1999) Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J Neurocytol 28:303–318
Bindels RJ (1993) Calcium handling by the mammalian kidney. J Exp Biol 184:89–104
Bodding M, Flockerzi V (2004) Ca2+ dependence of the Ca2+-selective TRPV6 channel. J Biol Chem 279:36546–36552
Boulay G (2002) Ca2+-calmodulin regulates receptor-operated Ca2+ entry activity of TRPC6 in HEK-293 cells. Cell Calcium 32:201–207
Bro S, Olgaard K (1997) Effects of excess PTH on nonclassical target organs. Am J Kidney Dis 30:606–620
Bronner F (2003) Mechanisms of intestinal calcium absorption. J Cell Biochem 88:387–393
Bronner F, Pansu D, Stein WD (1986) An analysis of intestinal calcium transport across the rat intestine. Am J Physiol Cell 250:G561–G569
Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC (1993) Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366:575–580
Chang Q, Gyftogianni E, van de Graaf SF, Hoefs S, Weidema FA, Bindels RJ, Hoenderop JG (2004) Molecular determinants in TRPV5 channel assembly. J Biol Chem 279:54304–54311
Clapham DE, Runnels LW, Strubing C (2001) The TRP ion channel family. Nat Rev Neurosci 2:387–396
Colin EM, van den Bemd GJ, van Aken M, Christakos S, de Jonge HR, Deluca HF, Prahl JM, Birkenhager JC, Buurman CJ, Pols HA, van Leeuwen JP (1999) Evidence for involvement of 17beta-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the rat. J Bone Miner Res 14:57–64
Costanzo LS, Windhager EE (1978) Calcium and sodium transport by the distal convoluted tubule of the rat. Am J Physiol Renal Physiol 235:F492–F506
Dardenne O, Prud’homme J, Arabian A, Glorieux FH, St-Arnaud R (2001) Targeted inactivation of the 25-hydroxyvitamin D3−1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology 142:3135–3141
Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW (1998) Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 58:1515–1520
Ellison DH (2000) Divalent cation transport by the distal nephron: insights from Bartter’s and Gitelman’s syndromes. Am J Physiol Renal Physiol 279:F616–F625
Embark H, Setiawan I, Poppendieck S, van de Graaf S, Boehmer C, Palmada M, Wieder T, Gerstberger R, Cohen P, Yun C, Bindels R, Lang F (2004) Regulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase isoforms SGK1 and SGK3 expressed in Xenopus oocytes. Cell Physiol Biochem 14:203–212
Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA (2004) Ca2+-selective TRPV channel architecture and function require a specific ankyrin repeat. J Biol Chem 279:34456–34463
Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H (2003) Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene 22:7858–7861
Fleet JC, Eksir F, Hance KW, Wood RJ (2002) Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am J Physiol Gastrointest Liver Physiol 283:G618–G625
Frick KK, Bushinsky DA (2003) Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol 14:1082–1095
Friedman PA (1998) Codependence of renal calcium and sodium transport. Annu Rev Physiol 60:179–197
Friedman PA, Bushinsky DA (1999) Diuretic effects on calcium metabolism. Semin Nephrol 19:551–556
Friedman PA, Gesek FA (1995) Cellular calcium transport in renal epithelia: measurement, mechanisms, and regulation. Physiol Rev 75:429–471
Fukugawa M, Kurokawa K (2002) Calcium homeostasis and imbalance. Nephron 92:41–45
Gennari C, Agnusdei D, Nardi P, Civitelli R (1990) Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J Clin Endocrinol Metab 71:1288–1293
Gerke V, Moss SE (2002) Annexins: from structure to function. Physiol Rev 82:331–371
Gkika D, Mahieu F, Nilius B, Hoenderop JG, Bindels RJ (2004) 80K-H as a new Ca2+ sensor regulating the activity of the epithelial Ca2+ channel transient receptor potential cation channel V5 (TRPV5). J Biol Chem 279:26351–26357
Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB (2000) Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342:1478–1483
Gunthorpe MJ, Benham CD, Randall A, Davis JB (2002) The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci 23:183–191
Harteneck C, Plant TD, Schultz G (2000) From worm to man: three subfamilies of TRP channels. Trends Neurosci 23:159–166
Haverstick DM, Heady TN, Macdonald TL, Gray LS (2000) Inhibition of human prostate cancer proliferation in vitro and in a mouse model by a compound synthesized to block Ca2+ entry. Cancer Res 60:1002–1008
Heller HJ, Sakhaee K, Moe OW, Pak CY (2002) Etiological role of estrogen status in renal stone formation. J Urol 168:1923–1927
Hirai M, Shimizu N (1990) Purification of two distinct proteins of approximate Mr 80,000 from human epithelial cells and identification as proper substrates for protein kinase C. Biochem J 270:583–589
Hirnet D, Olausson J, Fecher-Trost C, Bodding M, Nastainczyk W, Wissenbach U, Flockerzi V, Freichel M (2003) The TRPV6 gene, cDNA and protein. Cell Calcium 33:509–518
Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ (1999) Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 274:8375–8378
Hoenderop JG, Hartog A, Stuiver M, Doucet A, Willems PH, Bindels RJ (2000a) Localization of the epithelial Ca2+ channel in rabbit kidney and intestine. J Am Soc Nephrol 11:1171–1178
Hoenderop JG, Muller D, Suzuki M, van Os CH, Bindels RJ (2000b) Epithelial calcium channel: gate-keeper of active calcium reabsorption. Curr Opin Nephrol Hypertens 9:335–340
Hoenderop JG, Muller D, van der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, van Os CH, Bindels RJ (2001a) Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol 12:1342–1349
Hoenderop JG, Vennekens R, Muller D, Prenen J, Droogmans G, Bindels RJ, Nilius B (2001b) Function and expression of the epithelial Ca2+ channel family: comparison of the mammalian epithelial Ca2+ channel 1 and 2. J Physiol 537:747–761
Hoenderop JG, Dardenne O, van Abel M, van der Kemp AW, van Os CH, St-Arnaud R, Bindels RJ (2002a) Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1α-hydroxylase knockout mice. FASEB J 16:1398–1406
Hoenderop JG, Nilius B, Bindels RJ (2002b) Molecular mechanisms of active Ca2+ reabsorption in the distal nephron. Annu Rev Physiol 64:529–549
Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ (2003a) Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112:1906–1914
Hoenderop JG, Voets T, Hoefs S, Weidema AF, Prenen J, Nilius B, Bindels RJ (2003b) Homo- and heterotetrameric architecture of the epithelial Ca2+ channels, TRPV5 and TRPV6. EMBO J 22:776–785
Hoenderop JG, van der Kemp AW, Urben CM, Strugnell SA, Bindels RJ (2004) Effects of vitamin D compounds on renal and intestinal Ca2+ transport proteins in 25-hydroxyvitamin D3-1α-hydroxylase knockout mice. Kidney Int 66:1082–1089
Hoenderop JG, Nilius B, Bindels RJ (2005) Calcium absorption across epithelia. Physiol Rev 85:373–422
Hughes MR, Malloy PJ, Kieback DG, Kesterson RA, Pike JW, Feldman D, O’Malley BW (1988) Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science 242:1702–1705
Hung AY, Sheng M (2002) PDZ domains: structural modules for protein complex assembly. J Biol Chem 277:5699–5702
Hurwitz S (1996) Homeostatic control of plasma calcium concentration. Crit Rev Biochem Mol Biol 31:41–100
Janssen SW, Hoenderop JG, Hermus AR, Sweep CG, Martens GJ, Bindels RJ (2002) Expression of the novel epithelial Ca2+ channel ECaC in rat pancreatic islets. J Histochem Cytochem 50:789–798
Khosla S, Ebeling PR, Firek AF, Burritt MM, Kao PC, Heath H III (1993) Calcium infusion suggests a “set-point” abnormality of parathyroid gland function in familial benign hypercalcemia and more complex disturbances in primary hyperparathyroidism. J Clin Endocrinol Metab 76:715–720
Kitanaka S, Takeyama K, Murayama A, Sato T, Okumura K, Nogami M, Hasegawa Y, Niimi H, Yanagisawa J, Tanaka T, Kato S (1998) Inactivating mutations in the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N Engl J Med 338:653–661
Knutson JC, Hollis BW, LeVan LW, Valliere C, Gould KG, Bishop CW (1995) Metabolism of 1alpha-hydroxyvitamin D2 to activated dihydroxyvitamin D2 metabolites decreases endogenous 1alpha, 25-dihydroxyvitamin D3 in rats and monkeys. Endocrinology 136:4749–4753
Knutson JC, LeVan LW, Valliere CR, Bishop CW (1997) Pharmacokinetics and systemic effect on calcium homeostasis of 1alpha,24-dihydroxyvitamin D2 in rats. Comparison with 1alpha,25-dihydroxyvitamin D2, calcitriol, and calcipotriol. Biochem Pharmacol 53:829–837
Lambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ (2004) Regulation of the mouse epithelial Ca2+ channel TRPV6 by the Ca2+-sensor calmodulin. J Biol Chem 279:28855–28861
Lemmink HH, van den Heuvel LP, van Dijk HA, Merkx GF, Smilde TJ, Taschner PE, Monnens LA, Hebert SC, Knoers NV (1996) Linkage of Gitelman syndrome to the thiazide-sensitive sodium-chloride cotransporter gene with identification of mutations in Dutch families. Pediatr Nephrol 10:403–407
Lerolle N, Coulet F, Lantz B, Paillard F, Houillier P, Soubrier F, Gattegno B, Jeunemaitre X, Ronco P, Rondeau E (2001) No evidence for point mutations of the calcium-sensing receptor in familial idiopathic hypercalciuria. Nephrol Dial Transplant 16:2317–2322
Liu G, Oettel K, Ripple G, Staab MJ, Horvath D, Alberti D, Arzoomanian R, Marnocha R, Bruskewitz R, Mazess R, Bishop C, Bhattacharya A, Bailey H, Wilding G (2002) Phase I trial of 1alpha-hydroxyvitamin D2 in patients with hormone refractory prostate cancer. Clin Cancer Res 8:2820–2827
Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B (2001) Organization of the mouse distal nephron: distributions of transcellular calcium and sodium transport pathways. Am J Physiol Renal Physiol 281:F1021–F1027
Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, Bloch-Faure M, Hoenderop JG, Shull GE, Meneton P, Kaissling B (2004) Altered renal distal tubule structure and renal Na+ and Ca2+ handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol 15:2276–2288
Mery L, Strauss B, Dufour JF, Krause KH, Hoth M (2002) The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci 115:3497–3508
Miller GJ, Stapleton GE, Ferrara JA, Lucia MS, Pfister S, Hedlund TE, Upadhya P (1992) The human prostatic carcinoma cell line LNCaP expresses biologically active, specific receptors for 1 alpha,25-dihydroxyvitamin D3. Cancer Res 52:515–520
Miller LA, Stapleton FB (1989) Urinary volume in children with urolithiasis. J Urol 141:918–920
Monroy A, Plata C, Hebert SC, Gamba G (2000) Characterization of the thiazide-sensitive Na+–Cl− cotransporter: a new model for ions and diuretics interaction. Am J Physiol Renal Physiol 279:F161–F169
Montell C, Birnbaumer L, Flockerzi V (2002a) The TRP channels, a remarkably functional family. Cell 108:595–598
Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX (2002b) A unified nomenclature for the superfamily of TRP cation channels. Mol Cell 9:229–231
Mosavi LK, Minor DL Jr, Peng Z (2002) Consensus-derived structural determinants of the ankyrin repeat motif. Physiol Rev 99:16029–16034
Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY (2004) The ankyrin repeat as molecular architecture for protein recognition. Protein Sci 13:1435–1448
Muller D, Hoenderop JG, Meij IC, van den Heuvel LP, Knoers NV, den Hollander AI, Eggert P, Garcia-Nieto V, Claverie-Martin F, Bindels RJ (2000a) Molecular cloning, tissue distribution, and chromosomal mapping of the human epithelial Ca2+ channel (ECAC1). Genomics 67:48–53
Muller D, Hoenderop JG, Merkx GF, van Os CH, Bindels RJ (2000b) Gene structure and chromosomal mapping of human epithelial calcium channel. Biochem Biophys Res Commun 275:47–52
Muller D, Hoenderop JG, Vennekens R, Eggert P, Harangi F, Mehes K, Garcia-Nieto V, Claverie-Martin F, van Os C, Nilius B, Bindels RJ (2002) Epithelial Ca2+ channel (ECaC) in autosomal dominant idiopathic hypercalciuria. Nephrol Dial Transplant 17:1614–1620
Nemeth EF (1996) Calcium receptors as novel drug targets. In: Principles of bone biology. Academic, San Diego, pp 1019–1035
Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, Balandrin MF (1998) Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA 95:4040–4045
Nicotera P, Orrenius S (1998) The role of calcium in apoptosis. Cell Calcium 23:173–180
Niemeyer BA, Bergs C, Wissenbach U, Flockerzi V, Trost C (2001) Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc Natl Acad Sci USA 98:3600–3605
Nijenhuis T, Hoenderop JG, Loffing J, van der Kemp AW, van Os CH, Bindels RJ (2003) Thiazide-induced hypocalciuria is accompanied by a decreased expression of Ca2+ transport proteins in kidney. Kidney Int 64:555–564
Nijenhuis T, Hoenderop JG, Bindels RJ (2004) Downregulation of Ca2+ and Mg2+ transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol 15:549–557
Nilius B, Prenen J, Vennekens R, Hoenderop JG, Bindels RJ, Droogmans G (2001a) Modulation of the epithelial calcium channel, ECaC, by intracellular Ca2+. Cell Calcium 29:417–428
Nilius B, Vennekens R, Prenen J, Hoenderop JG, Droogmans G, Bindels RJ (2001b) The single pore residue D542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J Biol Chem 276:1020–1025
Nilius B, Prenen J, Hoenderop JG, Vennekens R, Hoefs S, Weidema AF, Droogmans G, Bindels RJ (2002) Fast and slow inactivation kinetics of the Ca2+ channels ECaC1 and ECaC2 (TRPV5 and 6): role of the intracellular loop located between transmembrane segment 2 and 3. J Biol Chem 277:30852–30858
Nilius B, Weidema F, Prenen J, Hoenderop JG, Vennekens R, Hoefs S, Droogmans G, Bindels RJ (2003) The carboxyl terminus of the epithelial Ca2+ channel ECaC1 is involved in Ca2+-dependent inactivation. Pflugers Arch 445:584–588
Nordin BE, Need AG, Morris HA, Horowitz M, Robertson WG (1991) Evidence for a renal calcium leak in postmenopausal women. J Clin Endocrinol Metab 72:401–407
Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M (2003) Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA 100:8002–8006
Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D (2004) Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem 279:16754–16766
Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D (1994) Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res 54:805–810
Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA (1999) Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 274:22739–22746
Peng JB, Chen XZ, Berger UV, Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA (2000) Human calcium transport protein CaT1. Biochem Biophys Res Commun 278:326–332
Peng JB, Brown EM, Hediger MA (2001a) Structural conservation of the genes encoding CaT1, CaT2, and related cation channels. Genomics 76:99–109
Peng JB, Zhuang L, Berger UV, Adam RM, Williams BJ, Brown EM, Hediger MA, Freeman MR (2001b) CaT1 expression correlates with tumor grade in prostate cancer. Biochem Biophys Res Commun 282:729–734
Peng JB, Brown EM, Hediger MA (2003) Epithelial Ca2+ entry channels: transcellular Ca2+ transport and beyond. J Physiol 551:729–740
Phillips AM, Bull A, Kelly LE (1992) Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron 8:631–642
Pollak MR, Brown EM, Chou YH, Hebert SC, Marx SJ, Steinman B, Levi T, Seidman SE, Seidman JG (1993) Mutations in the human Ca2+ sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 75:1297–1303
Pollak MR, Brown EM, Estep HL, McLaine PN, Kifor O, Park J, Hebert SC, Seidman CE, Seidman JG (1994) Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation. Nat Genet 8:303–307
Prince RL (1994) Counterpoint: estrogen effects on calcitropic hormones and calcium homeostasis. Endocr Rev 15:301–309
Prince RL, Smith M, Dick IM, Price RI, Webb PG, Henderson NK, Harris MM (1991) Prevention of postmenopausal osteoporosis. A comparative study of exercise, calcium supplementation, and hormone-replacement therapy. N Engl J Med 325:1189–1195
Rabinovitch A, Suarez-Pinzon WL, Sooy K, Strynadka K, Christakos S (2001) Expression of calbindin-D28K in a pancreatic islet beta-cell line protects against cytokine-induced apoptosis and necrosis. Endocrinology 142:3649–3655
Reichel H, Koeffler HP, Norman AW (1989) The role of the vitamin D endocrine system in health and disease. N Engl J Med 320:980–991
Reid IR (1997) Glucocorticoid osteoporosis—mechanisms and management. Eur J Endocrinol 137:209–217
Reid IR, Ibbertson HK (1987) Evidence for decreased tubular reabsorption of calcium in glucocorticoid-treated asthmatics. Horm Res 27:200–204
Reid IR, Ames RW, Orr-Walker BJ, Clearwater JM, Horne AM, Evans MC, Murray MA, McNeil AR, Gamble GD (2000) Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: a randomized controlled trial. Am J Med 109:362–370
Saimi Y, Kung C (2002) Calmodulin as an ion channels subunit. Annu Rev Physiol 64:289–311
Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE (1998) Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+–Cl− cotransporter of the distal convoluted tubule. J Biol Chem 273:29150–29155
Scott P, Ouimet D, Proulx Y, Trouve ML, Guay G, Gagnon B, Valiquette L, Bonnardeaux A (1998) The 1 alpha-hydroxylase locus is not linked to calcium stone formation or calciuric phenotypes in French–Canadian families. J Am Soc Nephrol 9:425–432
Shenolikar S, Weinman EJ (2001) NHERF: targeting and trafficking membrane proteins. Am J Physiol Renal Physiol 280:F389–F395
Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS (2002) Calmodulin regulates Ca2+-dependent feedback inhibition of store-operated Ca2+ influx by interaction with a site in the C terminus of TrpC1. Mol Cell 9:739–750
Slatopolsky E, Finch J, Brown A (2003) New vitamin D analogs. Kidney Int Suppl 63:83–87
Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S (2003) Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology 144:3885–3894
Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H (1994) Demographic and geographic variability of kidney stones in the United States. Kidney Int 46:893–899
Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX (2001) Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J Biol Chem 276:21303–21310
Trost C, Marquart A, Zimmer S, Philipp S, Cavalie A, Flockerzi V (1999) Ca2+-dependent interaction of the trpl cation channel and calmodulin. FEBS Lett 451:257–263
Trost C, Bergs C, Himmerkus N, Flockerzi V (2001) The transient receptor potential, TRP4, cation channel is a novel member of the family of calmodulin binding proteins. Biochem J 355:663–670
Tsavaler L, Shapero MH, Morkowski S, Laus R (2001) Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 61:3760–3769
Van Abel M, Hoenderop JG, Dardenne O, St-Arnaud R, van Os CH, van Leeuwen HJ, Bindels RJ (2002) 1,25-Dihydroxyvitamin D3-independent stimulatory effect of estrogen on the expression of ECaC1 in the kidney. J Am Soc Nephrol 13:2102–2109
Van Abel M, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ (2003) Regulation of the epithelial Ca2+ channels in small intestine as studied by quantitative mRNA detection. Am J Physiol Gastrointest Liver Physiol 285:G78–G85
Van Cromphaut S, Dewerchin M, Hoenderop JG, Stockmans I, van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G (2001) Active duodenal calcium absorption in vitamin D receptor-knock out mice: functional and molecular aspects. Proc Natl Acad Sci USA 98:13324–13329
Van de Graaf SF, Hoenderop JG, Gkika D, Lamers D, Prenen J, Rescher U, Gerke V, Staub O, Nilius B, Bindels RJ (2003) Functional expression of the epithelial Ca2+ channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J 22:1478–1487
Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ (2000) Permeation and gating properties of the novel epithelial Ca2+ channel. J Biol Chem 275:3963–3969
Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G, Nilius B (2001) Pore properties and ionic block of the rabbit epithelial calcium channel expressed in HEK 293 cells. J Physiol 530:183–191
Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B (2002) Current understanding of mammalian TRP homologues. Cell Calcium 31:253–264
Vezzoli G, Tanini A, Ferrucci L, Soldati L, Bianchin C, Franceschelli F, Malentacchi C, Porfirio B, Adamo D, Terranegra A, Falchetti A, Cusi D, Bianchi G, Brandi ML (2002) Influence of calcium-sensing receptor gene on urinary calcium excretion in stone-forming patients. J Am Soc Nephrol 13:2517–2523
Warr CG, Kelly LE (1996) Identification and characterization of two distinct calmodulin-binding sites in the Trpl ion-channel protein of Drosophila melanogaster. Biochem J 314:497–503
Wasserman RH, Fullmer CS (1995) Vitamin D and intestinal calcium transport: facts, speculations and hypotheses. J Nutr 125:1971S–1979S
Weber K, Erben RG, Rump A, Adamski J (2001) Gene structure and regulation of the murine epithelial calcium channels ECaC1 and 2. Biochem Biophys Res Commun 289:1287–1294
Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, Reus K, Meese E, Bonkhoff H, Flockerzi V (2001) Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem 276:19461–19468
Wissenbach U, Niemeyer B, Himmerkus N, Fixemer T, Bonkhoff H, Flockerzi V (2004) TRPV6 and prostate cancer: cancer growth beyond the prostate correlates with increased TRPV6 Ca2+ channel expression. Biochem Biophys Res Commun 322:1359–1363
Wood RJ, Tchack L, Taparia S (2001) 1,25-Dihydroxyvitamin D3 increases the expression of the CaT1 epithelial calcium channel in the Caco-2 human intestinal cell line. BMC Physiol 1:11
Young MM, Nordin BE (1969) The effect of the natural and artificial menopause on bone density and fracture. Proc R Soc Med 62:242
Yun CC, Palmada M, Embark HM, Fedorenko O, Feng Y, Henke G, Setiawan I, Boehmer C, Weinman EJ, Sandrasagra S, Korbmacher C, Cohen P, Pearce D, Lang F (2002) The serum and glucocorticoid-inducible kinase SGK1 and the Na+/H+ exchange regulating factor NHERF2 synergize to stimulate the renal outer medullary K+ channel ROMK1. J Am Soc Nephrol 13:2823–2830
Zerwekh JE, Hughes MR, Reed BY, Breslau NA, Heller HJ, Lemke M, Nasonkin I, Pak CY (1995) Evidence for normal vitamin D receptor messenger ribonucleic acid and genotype in absorptive hypercalciuria. J Clin Endocrinol Metab 80:2960–2965
Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu MX (2001) Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci USA 98:3168–3173
Zhao XY, Peehl DM, Navone NM, Feldman D (2000) 1alpha,25-Dihydroxyvitamin D3 inhibits prostate cancer cell growth by androgen-dependent and androgen-independent mechanisms. Endocrinology 141:2548–2556
Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR (2002) Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Invest 82:1755–1764
Acknowledgements
This work was supported in part by grants from the Dutch Kidney Foundation (C10.1881) and the Dutch Organization of Scientific Research (Zon-Mw 902.18.298, Zon-Mw 016.006.001 NWO-ALW 805.09.042 and NWO-ALW 810.38.004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Abel, M., Hoenderop, J.G.J. & Bindels, R.J.M. The epithelial calcium channels TRPV5 and TRPV6: regulation and implications for disease. Naunyn-Schmiedeberg's Arch Pharmacol 371, 295–306 (2005). https://doi.org/10.1007/s00210-005-1021-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-005-1021-2