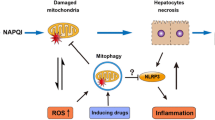
Abstract
Acetaminophen (APAP)-induced liver injury is an important clinical and toxicological problem. Understanding the mechanisms and modes of cell death are vital for the development of therapeutic interventions. The histological and clinical features of APAP hepatotoxicity including cell and organelle swelling, karyolysis, and extensive cell contents release lead to the characterization of the cell death as oncotic necrosis. However, the more recent identification of detailed signaling mechanisms of mitochondrial dysfunction, the amplification mechanisms of mitochondrial oxidant stress and peroxynitrite formation by a mitogen-activated protein kinase cascade, mechanisms of the mitochondrial permeability transition pore opening and nuclear DNA fragmentation as well as the characterization of the sterile inflammatory response suggested that the mode of cell death is better termed programmed necrosis. Additional features like mitochondrial Bax translocation and cytochrome c release, mobilization of lysosomal iron and the activation of receptor-interacting protein kinases and the inflammasome raised the question whether other emerging modes of cell death such as apoptosis, necroptosis, ferroptosis and pyroptosis could also play a role. The current review summarizes the key mechanisms of APAP-induced liver injury and compares these with key features of the newly described modes of cell death. Based on the preponderance of experimental and clinical evidence, the mode of APAP-induced cell death should be termed programmed necrosis; despite some overlap with other modes of cell death, APAP hepatotoxicity does not fulfill the characteristics of either apoptosis, necroptosis, ferroptosis, pyroptosis or autophagic cell death.



Similar content being viewed by others
Abbreviations
- AIF:
-
Apoptosis-inducing factor
- AIM2:
-
Absent in melanoma 2
- APAF-1:
-
Apoptotic protease-activating factor-1
- APAP:
-
Acetaminophen
- ASK1:
-
Apoptosis signal-regulating kinase 1
- CAD:
-
Caspase-activted DNase
- DAMP:
-
Damage-associated molecular pattern
- DMSO:
-
Dimethylsulfoxide
- GFP:
-
Green fluorescence protein
- GPx:
-
Glutathione peroxidase
- GSK-3b:
-
Glycogen synthase kinase 3 beta
- HETEs:
-
Hydroxy-eicosatetraenoic acids
- IAP:
-
Inhibitor of apoptosis
- ICAD:
-
Inhibitor of caspase-activated DNase
- IL-1:
-
Interleukin-1
- JNK:
-
c-Jun N-terminal kinase
- LPO:
-
Lipid peroxidation
- MAPK:
-
Mitogen-activating protein kinase
- MKK4:
-
Mitogen-activated protein kinase kinase 4
- MLK3:
-
Mixed-lineage kinase 3
- MLKL:
-
Mixed lineage kinase domain-like protein
- MPTP:
-
Mitochondrial membrane permeability transition pore
- mtDNA:
-
Mitochondrial DNA
- Nalp3:
-
NACHT, LRR and PYD domain-containing protein
- NAPQI:
-
N-acetyl-p-benzoquinone imine
- NCOA4:
-
Nuclear receptor coactivator 4
- PAMP:
-
Pathogen-associated molecular pattern
- PINK1:
-
PTEN-induced kinase 1
- PTEN:
-
Phosphatase and tensin homolog
- RIPK:
-
Receptor-interacting protein kinase
- Smac/Diablo:
-
Second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI
- SQSTM1/p62:
-
Sequestosome 1/p62
- TLR:
-
Toll-like receptor
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- TFEB:
-
Transcription factor EB
References
Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA (2001) Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol 60:907–915
Bajt ML, Cover C, Lemasters JJ, Jaeschke H (2006) Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci 94:217–225
Bajt ML, Farhood A, Lemasters JJ, Jaeschke H (2008) Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther 324:8–14
Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H (2011) Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci 122:598–605
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7:99–109
Boess F, Bopst M, Althaus R, Polsky S, Cohen SD, Eugster HP, Boelsterli UA (1998) Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology 27:1021–1029
Chao X, Wang H, Jaeschke H, Ding WX (2018) Role and mechanisms of autophagy in acetaminophen-induced liver injury. Liver Int 38:1363–1374
Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H (2005) Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther 315:879–887
Dara L, Johnson H, Suda J, Win S, Gaarde W, Han D, Kaplowitz N (2015) Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology 62:1847–1857
Deas E, Plun-Favreau H, Gandhi S, Desmond H, Kjaer S, Loh SH, Renton AE, Harvey RJ, Whitworth AJ, Martins LM, Abramov AY, Wood NW (2011) PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum Mol Genet 20:867–879
Degterev A, Maki JL, Yuan J (2013) Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ 20:366
Deutsch M, Graffeo CS, Rokosh R, Pansari M, Ochi A, Levie EM, Van Heerden E, Tippens DM, Greco S, Barilla R, Tomkötter L, Zambirinis CP, Avanzi N, Gulati R, Pachter HL, Torres-Hernandez A, Eisenthal A, Daley D, Miller G (2015) Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis 6:e1759
Doll S, Conrad M (2017) Iron and ferroptosis: a still ill-defined liaison. IUBMB Life 69:423–434
Du K, Xie Y, McGill MR, Jaeschke H (2015) Pathophysiological significance of c-Jun N-terminal kinase in acetaminophen hepatotoxicity. Expert Opin Drug Metab Toxicol 11:1769–1779
Du K, Ramachandran A, Jaeschke H (2016) Oxidative stress during acetaminophen hepatotoxicity: sources, pathophysiological role and therapeutic potential. Redox Biol 10:148–156
Du K, Ramachandran A, McGill MR, Mansouri A, Asselah T, Farhood A, Woolbright BL, Ding WX, Jaeschke H (2017) Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem Toxicol 108:339–350
Du K, Ramachandran A, Weemhoff JL, Woolbright BL, Jaeschke AH, Chao X, Ding WX, Jaeschke H (2019) Mito-tempo protects against acute liver injury but induces limited secondary apoptosis during the late phase of acetaminophen hepatotoxicity. Arch Toxicol 93:163–178
El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE (2003) Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol 191:118–129
Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R (1995) In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 21:1465–1468
Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H (2002) Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 67:322–328
Han D, Dara L, Win S, Than TA, Yuan L, Abbasi SQ, Liu ZX, Kaplowitz N (2013) Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharmacol Sci 34:243–253
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, Tang D (2016) Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12:1425–1428
Hu B, Colletti LM (2010) CXC receptor-2 knockout genotype increases X-linked inhibitor of apoptosis protein and protects mice from acetaminophen hepatotoxicity. Hepatology 52:691–702
Hu J, Kholmukhamedov A, Lindsey CC, Beeson CC, Jaeschke H, Lemasters JJ (2016) Translocation of iron from lysosomes to mitochondria during acetaminophen-induced hepatocellular injury: protection by starch-desferal and minocycline. Free Radic Biol Med 97:418–426
Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ (2009) Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Investig 119:305–314
Jaeschke H (2015) Acetaminophen: dose-dependent drug hepatotoxicity and acute liver failure in patients. Dig Dis 33:464–471
Jaeschke H, Lemasters JJ (2003) Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125:1246–1257
Jaeschke H, Kleinwaechter C, Wendel A (1987) The role of acrolein in allyl alcohol-induced lipid peroxidation and liver cell damage in mice. Biochem Pharmacol 36:51–57
Jaeschke H, Knight TR, Bajt ML (2003) The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett 144:279–288
Jaeschke H, Cover C, Bajt ML (2006) Role of caspases in acetaminophen-induced liver injury. Life Sci 78:1670–1676
Jaeschke H, Williams CD, Farhood A (2011) No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity. Hepatology 53:718–719
Jaeschke H, Duan L, Akakpo JY, Farhood A, Ramachandran A (2018) The role of apoptosis in acetaminophen hepatotoxicity. Food Chem Toxicol 118:709–718
Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R (2001) DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 61:1659–1665
Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ (2010) Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biology 191:933–942
Jorgensen I, Rayamajhi M, Miao EA (2017) Programmed cell death as a defense against infection. Nat Rev Immunol 17:151–164
Jouan-Lanhouet S, Riquet F, Duprez L, Vanden Berghe T, Takahashi N, Vandenabeele P (2014) Necroptosis, in vivo detection in experimental disease models. Semin Cell Dev Biol 35:2–13
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721
Knight TR, Jaeschke H (2002) Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: mitochondrial dysfunction versus glutathione depletion. Toxicol Appl Pharmacol 181:133–141
Knight TR, Fariss MW, Farhood A, Jaeschke H (2003) Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci 76:229–236
Kon K, Kim JS, Uchiyama A, Jaeschke H, Lemasters JJ (2010) Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol Sci 117:101–108
Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, Saeki Y, Tanaka K, Matsuda N (2014) Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510:162–166
Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM, Acute Liver Failure Study Group (2005) Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42:1364–1372
Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H (1999) Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol 156:179–186
Lee WM (2013) Drug-induced acute liver failure. Clin Liver Dis 17:575–586
Lei P, Bai T, Sun Y (2019) Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol 10:139
Li H, Wang P, Sun Q, Ding WX, Yin XM, Sobol RW, Stolz DB, Yu J, Zhang L (2011) Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of Beclin 1. Cancer Res 71:3625–3634
Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su Y, Shen YY, Chen Y, Xiong B, Yang CH, Ding J, Miao ZH (2014) The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis 5:e1278
Lőrincz T, Jemnitz K, Kardon T, Mandl J, Szarka A (2015) Ferroptosis is involved in acetaminophen induced cell death. Pathol Oncol Res 21:1115–1121
Malhi H, Gores GJ, Lemasters JJ (2006) Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43(2 Suppl 1):S31–S44
Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC (2014) Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509:105–109
Mathews WR, Guido DM, Fisher MA, Jaeschke H (1994) Lipid peroxidation as molecular mechanism of liver cell injury during reperfusion after ischemia. Free Radic Biol Med 16:763–770
Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189:211–221
McGill MR, Jaeschke H (2013) Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 30:2174–2187
McGill MR, Jaeschke H (2019) Animal models of drug-induced liver injury. Biochim Biophys Acta Mol Basis Dis 1865:1031–1039
McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H (2011) HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 53:974–982
McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H (2012) The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Investig 122:1574–1583
Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK (2011) The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem 117:856–867
Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M (2008) Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology 135:1311–1321
Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y (2009) Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nature reviews. Mol Cell Biol 10:458–467
Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ (2010) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8:e1000298
Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX (2012) Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology 55:222–232
Ni HM, Williams JA, Jaeschke H, Ding WX (2013) Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol 1:427–432
Ni HM, Williams JA, Ding WX (2015) Mitochondrial dynamics and mitochondrial quality control. Redox Biol 4:6–13
Ni HM, McGill MR, Chao X, Du K, Williams JA, Xie Y, Jaeschke H, Ding WX (2016) Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J Hepatol 65:354–362
Park Y, Smith RD, Combs AB, Kehrer JP (1988) Prevention of acetaminophen-induced hepatotoxicity by dimethyl sulfoxide. Toxicology 52:165–175
Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20:460–473
Pickles S, Vigie P, Youle RJ (2018) Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol 28:R170–R185
Ramachandran A, Jaeschke H (2019) Acetaminophen hepatotoxicity. Semin Liver Dis 39:221–234
Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H (2013) Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology 58:2099–2108
Ray SD, Sorge CL, Raucy JL, Corcoran GB (1990) Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol Appl Pharmacol 106:346–351
Ray SD, Kamendulis LM, Gurule MW, Yorkin RD, Corcoran GB (1993) Ca2+ antagonists inhibit DNA fragmentation and toxic cell death induced by acetaminophen. FASEB J 7:453–463
Ray SD, Mumaw VR, Raje RR, Fariss MW (1996) Protection of acetaminophen-induced hepatocellular apoptosis and necrosis by cholesteryl hemisuccinate pretreatment. J Pharmacol Exp Ther 279:1470–1483
Rojansky R, Cha MY, Chan DC (2016) Elimination of paternal mitochondria in mouse embryos occurs through autophagic degradation dependent on PARKIN and MUL1. Elife 5:e17896
Sakaida I, Kayano K, Wasaki S, Nagatomi A, Matsumura Y, Okita K (1995) Protection against acetaminophen-induced liver injury in vivo by an iron chelator, deferoxamine. Scand J Gastroenterol 30:61–67
Schnellmann JG, Pumford NR, Kusewitt DF, Bucci TJ, Hinson JA (1999) Deferoxamine delays the development of the hepatotoxicity of acetaminophen in mice. Toxicol Lett 106:79–88
Sharma M, Gadang V, Jaeschke A (2012) Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol 82:1001–1007
Shen W, Kamendulis LM, Ray SD, Corcoran GB (1991) Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: correlation of nuclear Ca2+ accumulation and early DNA fragmentation with cell death. Toxicol Appl Pharmacol 111:242–254
Shen W, Kamendulis LM, Ray SD, Corcoran GB (1992) Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: effects of Ca(2+)-endonuclease, DNA repair, and glutathione depletion inhibitors on DNA fragmentation and cell death. Toxicol Appl Pharmacol 112:32–40
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F (2014) Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514:187–192
Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, Kaplowitz N (2010) Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem 285:8244–8255
Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR (2014) Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136:4551–4556
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G (2019) The molecular machinery of regulated cell death. Cell Res 29:347–364
Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P (1998) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 187:1477–1485
Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrané J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S (2010) PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A 107:378–383
Wang LM, Cho YL, Tang YC, Wang JG, Park JE, Wu YJ, Wang CX, Tong Y, Chawla R, Zhang JB, Shi Y, Deng S, Lu G, Wu YH, Tan HWS, Pawijit P, Lim GGY, Chan HY, Zhang JZ, Fang L, Yu H, Liou YC, Karthik M, Bay BH, Lim KL, Sze SK, Yap CT, Shen HM (2018) PTEN-L is a novel protein phosphatase for ubiquitin dephosphorylation to inhibit PINK1-Parkin-mediated mitophagy. Cell Res 28:787–802
Wang H, Ni HM, Chao X, Ma X, Rodriguez YA, Chavan H, Wang S, Krishnamurthy P, Dobrowsky R, Xu DX, Jaeschke H, Ding WX (2019) Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice. Redox Biol 22:101148
Wendel A, Feuerstein S (1981) Drug-induced lipid peroxidation in mice–I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol 30:2513–2520
Wendel A, Jaeschke H, Gloger M (1982) Drug-induced lipid peroxidation in mice–II. Protection against paracetamol-induced liver necrosis by intravenous liposomally entrapped glutathione. Biochem Pharmacol 31:3601–3605
Williams JA, Ding WX (2018) Mechanisms, pathophysiological roles and methods for analyzing mitophagy—recent insights. Biol Chem 399:147–178
Williams CD, Farhood A, Jaeschke H (2010) Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol Appl Pharmacol 247:169–178
Williams CD, Antoine DJ, Shaw PJ, Benson C, Farhood A, Williams DP, Kanneganti TD, Park BK, Jaeschke H (2011) Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol Appl Pharmacol 252:289–297
Williams JA, Ni HM, Haynes A, Manley S, Li Y, Jaeschke H, Ding WX (2015) Chronic deletion and acute knockdown of Parkin have differential responses to acetaminophen-induced mitophagy and liver injury in mice. J Biol Chem 290:10934–10946
Win S, Than TA, Han D, Petrovic LM, Kaplowitz N (2011) c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem 286:35071–35078
Woolbright BL, Jaeschke H (2017) Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J Hepatol 66:836–848
Woolbright BL, Ramachandran A, McGill MR, Yan HM, Bajt ML, Sharpe MR, Lemasters JJ, Jaeschke H (2012) Lysosomal instability and cathepsin B release during acetaminophen hepatotoxicity. Basic Clin Pharmacol Toxicol 111:417–425
Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H (2014) Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol 279:266–274
Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, Jaeschke H (2015) Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol 286:1–9
Yamano K, Youle RJ (2013) PINK1 is degraded through the N-end rule pathway. Autophagy 9:1758–1769
Yang X, Chao X, Wang ZT, Ding WX (2016) The end of RIPK1-RIPK3-MLKL-mediated necroptosis in acetaminophen-induced hepatotoxicity? Hepatology 64:311–312
Yonekawa T, Gamez G, Kim J, Tan AC, Thorburn J, Gump J, Thorburn A, Morgan MJ (2015) RIP1 negatively regulates basal autophagic flux through TFEB to control sensitivity to apoptosis. EMBO Rep 16:700–708
Yuan J, Najafov A, Py BF (2016) Roles of caspases in necrotic cell death. Cell 167:1693–1704
Zhang YF, He W, Zhang C, Liu XJ, Lu Y, Wang H, Zhang ZH, Chen X, Xu DX (2014) Role of receptor interacting protein (RIP)1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicol Lett 225:445–453
Zhang J, Min RWM, Le K, Zhou S, Aghajan M, Than TA, Win S, Kaplowitz N (2017) The role of MAP2 kinases and p38 kinase in acute murine liver injury models. Cell Death Dis 8:e2903
Zhang C, Feng J, Du J, Zhuo Z, Yang S, Zhang W, Wang W, Zhang S, Iwakura Y, Meng G, Fu YX, Hou B, Tang H (2018) Macrophage-derived IL-1α promotes sterile inflammation in a mouse model of acetaminophen hepatotoxicity. Cell Mol Immunol 15:973–982
Acknowledgements
This work was supported in part by National Institutes of Health Grants R01 DK102142, R01 DK070195, P20 GM103549 and P30 GM118247.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H Jaeschke received grant support from McNeil Consumer Health, Inc. All the other authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jaeschke, H., Ramachandran, A., Chao, X. et al. Emerging and established modes of cell death during acetaminophen-induced liver injury. Arch Toxicol 93, 3491–3502 (2019). https://doi.org/10.1007/s00204-019-02597-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-019-02597-1