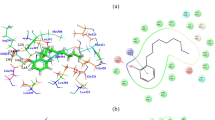
Abstract
Certain bisphenols (BPs) have been regarded as endocrine-disrupting chemicals due to their structural similarities to bisphenol A (BPA), a well-known weak estrogenic chemical. However, very limited data are currently available on the relationship between estrogenic activity and the structure of BP analogs. Therefore, we systematically investigated the estrogenic potency of 14 selected BP analogs with typical structures using experimental and computational methods. Most of the tested BP analogs exhibited weak estrogenic activities in both cell proliferation and MVLN assays with the exception of TBBPA, TCBPA and TBBPS. Molecular modeling techniques have been performed to investigate the dynamic structural characteristics of recognition processes between BPs and estrogen receptor α (ERα) at the atomic level. Thr347 was identified as the key residue responsible for the recognition of TBBPA, TCBPA and TBBPS by means of induced-fit H-bonding interactions in the binding pocket of ERα, whereas other BPs, in turn, rely on the alternative formation of H-bonds with His524. Subsequent allosteric modulation interferes significantly with the stability of helix 12 that is crucial for the transcriptional activity of ERα. These structural perturbations that are induced by the three compounds were further confirmed to reduce the recruitment potency of co-activators more than other BPs based on calculations of binding free energies, which is in line with observed experimental transcriptional activities. Our findings may help to elucidate the estrogenic potency of BPs with different molecular structures.








Similar content being viewed by others
References
Aarts JM, Wang S, Houtman R et al (2013) Robust array-based coregulator binding assay predicting ERα-Agonist potency and generating binding profiles reflecting ligand structure. Chem Res Toxicol 26(3):336–346
Anandakrishnan R, Aguilar B, Onufriev AV (2012) H++ 3.0: automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucl Acid Res 40(W1):W537–W541
Ando S, De Amicis F, Rago V et al (2002) Breast cancer: from estrogen to androgen receptor. Mol Cell Endocrinol 193(1):121–128
Andrianou XD, Gängler S, Piciu A, Charisiadis P, Zira C, Aristidou K, Piciu D, Hauser R, Makris KC (2016) Human exposures to bisphenol A, bisphenol F and chlorinated bisphenol a derivatives and thyroid function. PLoS One 11(10):e0155237
Berendsen HJ, Postma JPM, van Gunsteren WF, DiNola A, Haak J (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81(8):3684–3690
Bourgoin-Voillard S, Gallo D, Laïos I et al (2010) Capacity of type I and II ligands to confer to estrogen receptor alpha an appropriate conformation for the recruitment of coactivators containing a LxxLL motif—relationship with the regulation of receptor level and ERE-dependent transcription in MCF-7 cells. Biochem Pharmacol 79(5):746–757
Bramlett KS, Burris TP (2002) Effects of selective estrogen receptor modulators (SERMs) on coactivator nuclear receptor (NR) box binding to estrogen receptors. Mol Genet Metab 76(3):225–233
Bruning JB, Parent AA, Gil G et al (2010) Coupling of receptor conformation and ligand orientation determine graded activity. Nat Chem Biol 6(11):837–843
Brzozowski AM, Pike AC, Dauter Z et al (1997) Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389(6652):753–758
Cao H, Sun Y, Wang L, Zhao C, Fu J, Zhang A (2017) Understanding microscopic binding mechanism of hydroxylated and sulfated polybrominated diphenyl ethers with transthyretin by molecular docking, molecular dynamics simulations and binding free energy calculations. Mol Biosyst 13:736–749
Carraz M, Zwart W, Phan T, Michalides R, Brunsveld L (2009) Perturbation of estrogen receptor α localization with synthetic nona-arginine LXXLL-peptide coactivator binding inhibitors. Chem Biol 16(7):702–711
Chang C, Norris JD, Grøn H et al (1999) Dissection of the LXXLL nuclear receptor-coactivator interaction motif using combinatorial peptide libraries: discovery of peptide antagonists of estrogen receptors α and β. Mol Cell Biol 19(12):8226–8239
Chen MY, Ike M, Fujita M (2002) Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol 17(1):80–86
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N·log (N) method for Ewald sums in large systems. J Chem Phys 98(12):10089–10092
Delfosse V, Grimaldi M, Pons JL et al (2012) Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc Natl Acad Sci USA 109(37):14930–14935
Delfosse V, Grimaldi M, Cavailles V, Balaguer P, Bourguet W (2014) Structural and functional profiling of environmental ligands for estrogen receptors. Environ Health Perspect 122(12):1306
Desaulniers D, Leingartner K, Zacharewski T, Foster W (1998) Optimization of an MCF7-E3 cell proliferation assay and effects of environmental pollutants and industrial chemicals. Toxicol In Vitro 12(4):409–422
Dickson RB, Lippman ME (1995) Growth factors in breast cancer. Endocr Rev 16(5):559–589
Diel IJ, Solomayer EF, Seibel MJ et al (1999) Serum bone sialoprotein in patients with primary breast cancer is a prognostic marker for subsequent bone metastasis. Clin Cancer Res 5(12):3914–3919
Fox T, Kollman PA (1998) Application of the RESP methodology in the parametrization of organic solvents. J Phys Chem B 102(41):8070–8079
Han C, Fang S, Cao H, Lu Y, Ma Y, Wei D, Xie X, Liu X, Fei D, Zhao C (2013) Molecular interaction of PCB153 to human serum albumin: insights from spectroscopic and molecular modeling studies. J Hazard Mater 248:313–321
Hanson RN, Hua E, Adam Hendricks J, Labaree D, Hochberg RB (2012) Synthesis and evaluation of 11β-(4-substituted phenyl) estradiol analogs: transition from estrogen receptor agonists to antagonists. Biorg Med Chem 20(12):3768–3780
Heery DM, Kalkhoven E, Hoare S, Parker MG (1997) A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387(6634):733–736
Henley DV, Korach KS (2006) Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology 147(6):s25–s32
Hou T, Wang J, Li Y, Wang W (2011a) Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J Chem Inf Model 51(1):69–82
Hou T, Wang J, Li Y, Wang W (2011b) Assessing the performance of the molecular mechanics/Poisson Boltzmann surface area and molecular mechanics/generalized Born surface area methods. II. The accuracy of ranking poses generated from docking. J Comput Chem 32(5):866–877
Jakobsson K, Thuresson K, Rylander L, Sjödin A, Hagmar L, Bergman Å (2002) Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere 46(5):709–716
Jeyakumar M, Carlson KE, Gunther JR, Katzenellenbogen JA (2011) Exploration of dimensions of estrogen potency parsing ligand binding and coactivator binding affinities. J Biol Chem 286(15):12971–12982
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79(2):926–935
Kitamura S, Suzuki T, Sanoh S et al (2005) Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci 84(2):249–259
Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, Green HF, Liu T, Chae PS, Dror RO, Shaw DE, Weis WI, Wess J, Kobilka BK (2012) Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482(7386):552–556
Li Y, Burns KA, Arao Y, Luh CJ, Korach KS (2012) Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro. Environ Health Perspect 120(7):1029–1035
Li Y, Luh CJ, Burns KA et al (2013) Endocrine-disrupting chemicals (EDCs). In vitro mechanism of estrogenic activation and differential effects on ER target genes. Environ Health Perspect 121(4):459–466
Liu H, An X, Li S, Wang Y, Li J, Liu H (2015) Interaction mechanism exploration of R-bicalutamide/S-1 with WT/W741L AR using molecular dynamics simulations. Mol Biosyst 11(12):3347–3354
Liu J, Li J, Wu Y, Zhao Y, Luo F, Li S, Yang L, Moez EK, Dinu I, Martin JW (2017) Bisphenol A metabolites and bisphenol S in paired maternal and cord serum. Environ Sci Technol 51(4):2456–2463
Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C (2015) ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput 11(8):3696–3713
Margeat E, Poujol N, Boulahtouf A et al (2001) The human estrogen receptor α dimer binds a single SRC-1 coactivator molecule with an affinity dictated by agonist structure. J Mol Biol 306(3):433–442
Metskas LA, Rhoades E (2015) Conformation and dynamics of the troponin I C-terminal domain: combining single-molecule and computational approaches for a disordered protein region. J Am Chem Soc 137(37):11962–11969
Miller BR III, McGee TD Jr, Swails JM, Homeyer N, Gohlke H, Roitberg AE (2012) MMPBSA. py: an efficient program for end-state free energy calculations. J Chem Theory Comput 8(9):3314–3321
Molina-Molina JM, Amaya E, Grimaldi M et al (2013) In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol 272(1):127–136
Mongan J, Simmerling C, McCammon JA, Case DA, Onufriev A (2007) Generalized Born model with a simple, robust molecular volume correction. J Chem Theory Comput 3(1):156–169
Ng HW, Shu M, Luo H et al (2015) Estrogenic activity data extraction and in silico prediction show the endocrine disruption potential of bisphenol a replacement compounds. Chem Res Toxicol 28(9):1784–1795
Onufriev A, Bashford D, Case DA (2004) Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins Struct Funct Bioinf 55(2):383–394
Osborne CK, Clemmons DR, Arteaga CL (1990) Regulation of breast cancer growth by insulin-like growth factors. J Steroid Biochem Mol Biol 37(6):805–809
Pike AC, Brzozowski AM, Walton J et al (2001) Structural insights into the mode of action of a pure antiestrogen. Structure 9(2):145–153
Ren XM, Zhang YF, Guo LH, Qin ZF, Lv QY, Zhang LY (2015) Structure–activity relations in binding of perfluoroalkyl compounds to human thyroid hormone T3 receptor. Arch Toxicol 89(2):233–242
Riu A, Grimaldi M, le Maire A et al (2011) Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ Health Perspect 119(9):1227–1232
Robinson DR, Wu YM, Vats P et al (2013) Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 45(12):1446–1451
Roe DR, Cheatham TE III (2013) PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J Chem Theory Comput 9(7):3084–3095
Rosenmai AK, Dybdahl M, Pedersen M et al (2014) Are structural analogues to bisphenol A safe alternatives? Toxicol Sci 139(1):35–47
Ruan T, Liang D, Song S, Song M, Wang H, Jiang G (2015) Evaluation of the in vitro estrogenicity of emerging bisphenol analogs and their respective estrogenic contributions in municipal sewage sludge in China. Chemosphere 124:150–155
Ryckaert JP, Ciccotti G, Berendsen HJ (1977) Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys 23(3):327–341
Salomon-Ferrer R, Götz AW, Poole D, Le GS, Walker RC (2013) Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J Chem Theory Comput 9(9):3878–3888
Sheng N, Li J, Liu H, Zhang A, Dai J (2016) Interaction of perfluoroalkyl acids with human liver fatty acid-binding protein. Arch Toxicol 90(1):217–227
Shiau AK, Barstad D, Loria PM et al (1998) The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95(7):927–937
Sindhikara DJ, Kim S, Voter AF, Roitberg AE (2009) Bad seeds sprout perilous dynamics: stochastic thermostat induced trajectory synchronization in biomolecules. J Chem Theory Comput 5(6):1624–1631
Singh T, Adekoya OA, Jayaram B (2015) Understanding the binding of inhibitors of matrix metalloproteinases by molecular docking, quantum mechanical calculations, molecular dynamics simulations, and a MMGBSA/MMBappl study. Mol BioSyst 11(4):1041–1051
Song M, Xu Y, Jiang Q et al (2006) Measurement of estrogenic activity in sediments from Haihe and Dagu River, China. Environ Int 32(5):676–681
Song M, Liang D, Liang Y et al (2014) Assessing developmental toxicity and estrogenic activity of halogenated bisphenol A on zebrafish (Danio rerio). Chemosphere 112:275–281
Teng C, Goodwin B, Shockley K et al (2013) Bisphenol A affects androgen receptor function via multiple mechanisms. Chem Biol Interact 203(3):556–564
van der Burg B, Rutteman GR, Blankenstein MA, de Laat SW, van Zoelen EJ (1988) Mitogenic stimulation of human breast cancer cells in a growth factor-defined medium: synergistic action of insulin and estrogen. J Cell Physiol 134(1):101–108
Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM (2007) Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology 148(1):116–127
Vom Saal FS, Akingbemi BT, Belcher SM et al (2007) Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol 24(2):131–138
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25(9):1157–1174
Wang Z, Sun H, Yao X et al (2016) Comprehensive evaluation of ten docking programs on a diverse set of protein–ligand complexes: the prediction accuracy of sampling power and scoring power. Phys Chem Chem Phys 18(18):12964–12975
Weiser J, Shenkin PS, Still WC (1999) Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J Comput Chem 20(2):217–230
Yang CZ, Yaniger SI, Jordan VC, Klein DJ, Bittner GD (2011) Most plastic products release estrogenic chemicals: a potential health problem that can be solved. Environ Health Perspect 119(7):989–996
Yang Y, Lv QY, Guo LH, Wan B, Ren XM, Shi YL, Cai YQ (2017) Identification of protein tyrosine phosphatase SHP-2 as a new target of perfluoroalkyl acids in HepG2 cells. Arch Toxicol 91:1697–1707
Zhao G, Perilla JR, Yufenyuy EL, Meng X, Chen B, Ning J, Ahn J, Gronenborn AM, Schulten K, Aiken C, Zhang P (2013) Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 497(7451):643–646
Acknowledgements
This work was jointly supported in part by Chinese Academy of Sciences (XDB14030500, YSW2013B01), the National Natural Science Foundation (21177146), the National High Technology Research and Development Program (863) of China (2013AA065201), and the State Key Laboratory of Microbial Technology Open Projects Fund (M2015-07).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors’ declares that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, H., Wang, F., Liang, Y. et al. Experimental and computational insights on the recognition mechanism between the estrogen receptor α with bisphenol compounds. Arch Toxicol 91, 3897–3912 (2017). https://doi.org/10.1007/s00204-017-2011-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-2011-0