Abstract
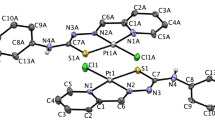
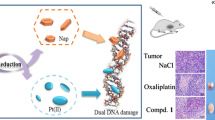
The therapeutic efficacy of the anticancer drug cisplatin is limited by the development of resistance. We therefore investigated newly synthesized platinum-nitroxyl complexes (PNCs) for their potential to circumvent cisplatin resistance. The complexes used were PNCs with bivalent cis-PtII(R·NH2)(NH3)Cl2 and cis-PtII(DAPO)Ox and four-valent platinum cis,trans,cis-PtIV(R·NH2)(NH3)(OR)2Cl2 and cis,trans,cis-PtIV(DAPO)(OR)2Ox, where R· are TEMPO or proxyl nitroxyl radicals, DAPO is trans-3,4-diamino-2,2,6,6-tetramethylpiperidine-1-oxyl, and OR and Ox are carboxylato and oxalato ligands, respectively. The complexes were characterized by spectroscopic methods, HPLC, log P ow data and elemental analysis. We studied intracellular platinum accumulation, DNA platination and cytotoxicity upon treatment with the PNCs in a model system of the bladder cancer cell line RT112 and its cisplatin-resistant subline RT112-CP. Platinum accumulation and DNA platination were similar in RT112 and RT112-CP cells for both bivalent and four-valent PNCs, in contrast to cisplatin for which a reduction in intracellular accumulation and DNA platination was observed in the resistant subline. The PNCs were found to platinate DNA in relation to the length of their axial RO-ligands. Furthermore, the PNCs were increasingly toxic in relation to the elongation of their axial RO-ligands, with similar toxicities in RT112 and its cisplatin-resistant subline. Using a cell-free assay, we observed induction of oxidative DNA damage by cisplatin but not PNCs suggesting that cisplatin exerts its toxic action by platination and oxidative DNA damage, while cells treated with PNCs are protected against oxidatively induced lesions. Altogether, our study suggests that PNCs may provide a more effective treatment for tumors which have developed resistance toward cisplatin.






Similar content being viewed by others
References
Andersson A, Fagerberg J, Lewensohn R, Ehrsson H (1996) Pharmacokinetics of cisplatin and its monohydrated complex in humans. J Pharm Sci 85:824–827
Asmuss M, Mullenders LH, Eker A, Hartwig A (2000) Differential effects of toxic metal compounds on the activities of Fpg and XPA, two zinc finger proteins involved in DNA repair. Carcinogenesis 21:2097–2104
Basu A, Krishnamurthy S (2010) Cellular responses to Cisplatin-induced DNA damage. J Nucleic Acids. doi:10.4061/2010/201367
Boiteux S, Bichara M, Fuchs RP, Laval J (1989) Excision of the imidazole ring-opened form of N-2-aminofluorene-C(8)-guanine adduct in poly(dG-dC) by Escherichia coli formamidopyrimidine-DNA glycosylase. Carcinogenesis 10:1905–1909
Bragado P, Armesilla A, Silva A, Porras A (2007) Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis 12:1733–1742
Brozovic A, Osmak M (2007) Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett 251:1–16
Cadet J, Wagner JR (2013) DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol 5:a012599
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378
Eadsforth C, Moser P (1983) Assessment of reverse-phase chromatographic methods for determining partition coefficients. Chemosphere 12:1459–1475
Giandomenico CM et al (1995) Carboxylation of kinetically inert platinum(IV) hydroxy complexes. An entrée into orally active platinum(IV) antitumor agents. Inorg Chem 34:1015–1021
Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM (2008) The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol 48:495–535
Hector S, Bolanowska-Higdon W, Zdanowicz J, Hitt S, Pendyala L (2001) In vitro studies on the mechanisms of oxaliplatin resistance. Cancer Chemother Pharmacol 48:398–406
Ishida S, Lee J, Thiele DJ, Herskowitz I (2002) Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA 99:14298–14302
Ishida S, McCormick F, Smith-McCune K, Hanahan D (2010) Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 17:574–583
Jandial DD, Farshchi-Heydari S, Larson CA, Elliott GI, Wrasidlo WJ, Howell SB (2009) Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin Cancer Res 15:553–560
Kelland L (2007) Broadening the clinical use of platinum drug-based chemotherapy with new analogues. Satraplatin and picoplatin. Expert Opin Investig Drugs 16:1009–1021
Kilari D, Guancial E, Kim ES (2016) Role of copper transporters in platinum resistance. World J Clin Oncol 7:106–113
Köberle B, Payne J, Grimaldi KA, Hartley JA, Masters JRW (1996) DNA-repair in cisplatin-sensitive and resistant human cell-lines measured in specific genes by quantitative polymerase chain-reaction. Biochem Pharmacol 52:1729–1734
Köberle B, Tomicic MT, Usanova S, Kaina B (2010) Cisplatin resistance: preclinical findings and clinical implications. Biochim Biophys Acta 1806:172–182
Koepsell H, Endou H (2004) The SLC22 drug transporter family. Pflugers Arch 447:666–676
Koga H, Kotoh S, Nakashima M, Yokomizo A, Tanaka M, Naito S (2000) Accumulation of intracellular platinum is correlated with intrinsic cisplatin resistance in human bladder cancer cell lines. Int J Oncol 16:1003–1007
Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T (2007) The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev 26:71–83
Kuo MT, Fu S, Savaraj N, Chen HH (2012) Role of the human high-affinity copper transporter in copper homeostasis regulation and cisplatin sensitivity in cancer chemotherapy. Cancer Res 72:4616–4621
Liang ZD et al (2012) Mechanistic basis for overcoming platinum resistance using copper chelating agents. Mol Cancer Ther 11:2483–2494
Loh SY, Mistry P, Kelland LR, Abel G, Harrap KR (1992) Reduced drug accumulation as a major mechanism of acquired resistance to cisplatin in a human ovarian carcinoma cell line: circumvention studies using novel platinum (II) and (IV) ammine/amine complexes. Br J Cancer 66:1109–1115
Marullo R et al (2013) Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 8:e81162
Masters JR et al (1986) Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines. Cancer Res 46:3630–3636
Masuda H, Tanaka T, Takahama U (1994) Cisplatin generates superoxide anion by interaction with DNA in a cell-free system. Biochem Biophys Res Commun 203:1175–1180
Mellish KJ, Kelland LR, Harrap KR (1993) In vitro drug chemosensitivity of human cervical squamous cell carcinoma cell lines with intrinsic and acquired resistance to cisplatin. Br J Cancer 68:240–250
OECD Guidelines for the Testing of Chemicals (2004) Test No 117: Partition coefficient (n-octanol/water); HPLC method. OECD Publishing, Paris
Oldenburg J et al (1994) Characterization of resistance mechanisms to cis-diamminedichloroplatinum(II) in three sublines of the CC531 colon adenocarcinoma cell line in vitro. Cancer Res 54:487–493
Pabla N, Huang S, Mi Q-S, Daniel R, Dong Z (2008) ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem 283:6572–6583
Pliss E, Sen V, Tichonov I (2013) Nitroxyl radicals in chemical and biological processes. LAP Lambert AP, Saarbrücken (in Russian)
Rabik CA, Dolan ME (2007) Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 33:9–23
Rixe O et al (1996) Oxaliplatin, tetraplatin, cisplatin and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug screen panel. Biochem Pharmacol 52:1855–1865
Samimi G, Howell SB (2006) Modulation of the cellular pharmacology of JM118, the major metabolite of satraplatin, by copper influx and efflux transporters. Cancer Chemother Pharmacol 57:781–788
Sen VD, Golubev VA, Volkova LM, Konovalova NP (1996) Synthesis and antitumor activity of platinum(II) complexes with trans-3,4-diamino-2,2,6,6-tetramethylpiperidine-1-oxyl. J Inorg Biochem 64:69–77
Sen VD et al (2000) Synthesis, structure, and biological activity of mixed-ligand platinum(II) complexes with aminonitroxides. Russ Chem Bull 49:1613–1619
Sen VD, Tkachev VV, Volkova LM, Goncharova SA, Raevskaya TA, Konovalova NP (2003) Synthesis, structure, and antitumor properties of platinum(IV) complexes with aminonitroxyl radicals. Russ Chem Bull 52:421–426
Sen VD, Goluvev A, Lugovskaya NY, Sashenkova TE, Konovolova NP (2006) Synthesis and antitumor properties of new platinum(IV) complexes with aminonitroxyl radicals. Russ Chem Bull 55:62–65
Sen VD, Terentiev AA, Konovalova NP (2012) Platinum complexes with bioactive nitroxyl radicals: synthesis and antitumor properties. In: Kokorin AI (ed) Nitroxides: theory, experiment and applications. InTech, Rijeka, pp 385–406
Shen DW, Goldenberg S, Pastan I, Gottesman MM (2000) Decreased accumulation of [14C]carboplatin in human cisplatin-resistant cells results from reduced energy-dependent uptake. J Cell Physiol 183:108–116
Siddik ZH (2003) Cisplatin: mode of action and molecular basis of resistance. Oncogene 22:7265–7279
Song IS et al (2004) Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther 3:1543–1549
Stewart DJ et al (1988) Platinum concentrations in human autopsy tumor samples. Am J Clin Oncol 11:152–158
Suy S, Mitchell JB, Samuni A, Mueller S, Kasid U (2005) Nitroxide tempo, a small molecule, induces apoptosis in prostate carcinoma cells and suppresses tumor growth in athymic mice. Cancer 103:1302–1313
Timmer-Bosscha H et al (1993) Cis-diamminedichloroplatinum(II) resistance in vitro and in vivo in human embryonal carcinoma-cells. Cancer Res 53:5707–5713
Walker MC, Povey S, Parrington JM, Riddle PN, Knuechel R, Masters JR (1990) Development and characterization of cisplatin-resistant human testicular and bladder tumour cell lines. Eur J Cancer 26:742–747
Wheate NJ, Walker S, Craig GE, Oun R (2010) The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans 39:8113–8127
Wilcox CS (2010) Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther 126:119–145
Yao X, Panichpisal K, Kurtzman N, Nugent K (2007) Cisplatin nephrotoxicity: a review. Am J Med Sci 334:115–124
Zisowsky J et al (2007) Relevance of drug uptake and efflux for cisplatin sensitivity of tumor cells. Biochem Pharmacol 73:298–307
Acknowledgments
High-resolution ESMS spectra were recorded in the Department of Structural Studies of Zelinsky Institute of Organic Chemistry, Moscow. The work was supported by Deutsche Forschungsgemeinschaft (DFG), Exzellenzinitiative KIT and Russian Scientific Fund (RSF) Grant No. 14-23-00018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Maria Cetraz and Vasily Sen have contributed equally to the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cetraz, M., Sen, V., Schoch, S. et al. Platinum(IV)-nitroxyl complexes as possible candidates to circumvent cisplatin resistance in RT112 bladder cancer cells. Arch Toxicol 91, 785–797 (2017). https://doi.org/10.1007/s00204-016-1754-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1754-3