Abstract
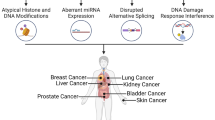
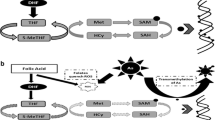
Arsenic is a human carcinogen with weak mutagenic properties that induces tumors through mechanisms not yet completely understood. People worldwide are exposed to arsenic-contaminated drinking water, and epidemiological studies showed a high percentage of lung, bladder, liver, and kidney cancer in these populations. Several mechanisms by which arsenical compounds induce tumorigenesis were proposed including genotoxic damage and chromosomal abnormalities. Over the past decade, a growing body of evidence indicated that epigenetic modifications have a role in arsenic-inducing adverse effects on human health. The main epigenetic mechanisms are DNA methylation in gene promoter regions that regulate gene expression, histone tail modifications that regulate the accessibility of transcriptional machinery to genes, and microRNA activity (noncoding RNA able to modulate mRNA translation). The “double capacity” of arsenic to induce mutations and epimutations could be the main cause of arsenic-induced carcinogenesis. The aim of this review is to better clarify the mechanisms of the initiation and/or the promotion of arsenic-induced carcinogenesis in order to understand the best way to perform an early diagnosis and a prompt prevention that is the key point for protecting arsenic-exposed population. Studies on arsenic-exposed population should be designed in order to examine more comprehensively the presence and consequences of these genetic/epigenetic alterations.



Similar content being viewed by others
References
Agency for Toxic Substances & Disease Registry Toxicological Profile for Arsenic (2013) http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=22&tid=3. Accessed 10 October 2013
Akram Z, Jalali S, Shami SA, Ahmad L, Batool S, Kalsoom O (2009) Genotoxicity of sodium arsenite and DNA fragmentation in ovarian cells of rat. Toxicol Lett 190:81–85. doi:10.1016/j.toxlet.2009.07.003
Akter KF, Owens G, Davey DE, Naidu R (2005) Arsenic speciation and toxicity in biological systems. Rev Environ Contam Toxicol 184:97–149
Alarifi S, Ali D, Alkahtani S, Siddiqui MA, Ali BA (2013) Arsenic trioxide-mediated oxidative stress and genotoxicity in human hepatocellular carcinoma cells. Onco Targets Ther 6:75–84. doi:10.2147/OTT.S38227
Aposhian HV (1997) Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Annu Rev Pharmacol Toxicol 37:397–419. doi:10.1149/annurev.pharmtox.37.1.397
Aposhian HV, Aposhian MM (2006) Arsenic toxicology: five questions. Chem Res Toxicol 9:1–15
Arguello RA, Cenget DD, Tello EE (1938) Cancer and arsenicism in the endemic region of Córdoba. Rev Argent Dermatofisiol 22:461–470
Avani G, Rao MV (2007) Genotoxic effects in human lymphocytes exposed to arsenic and vitamin A. Toxicol In Vitro 21:626–631
Baccarelli A, Ghosh S (2012) Environmental exposures, epigenetics and cardiovascular disease. Curr Opin Clin Nutr Metab Care 15(4):323–329. doi:10.1097/MCO.0b13e328354bf5c
Bagnyukova TV, Luzhna LI, Pogribny IP, Luschchak VI (2007) Oxidative stress and antioxidant defenses in goldfish liver in response to short-term exposure to arsenite. Environ Mol Mutagen 48:658–665
Bailey LB, Gregory JF 3rd (1999) Folate metabolism and requirements. J Nutr 129:779–782
Banerjee P, Biswas SJ, Belon P, Khuda-Bukhsh AR (2007) A potentized homeopathic drug, Arsenicum Album 200, can ameliorate genotoxicity induced by repeated injections of arsenic trioxide in mice. J Vet Med A Physiol Pathol Clin Med 54:370–376
Banerjee M, Sarma N, Biswas R, Roy J, Mukherjee A, Giri AK (2008) DNA repair deficiency leads to susceptibility to develop arsenic-induced premalignant skin lesions. Int J Cancer 123:283–287. doi:10.1002/ijc.23478
Bartel M, Ebert F, Leffers L, Karst U, Schwerdtle T (2011) Toxicological characterization of the inorganic and organic arsenic metabolite thio-DMA in cultured human lung cells. J Toxicol 2011:373141. doi:10.1155/2011/373141
Basu A, Mahata J, Roy AK, Sarkar JN, Poddar G, Nandy AK, Sarkar PK, Dutta PK, Banerjee A, Das M, Ray K, Roychaudhury S, Natarajan AT, Nilsson R, Giri AK (2002) Enhanced frequency of micronuclei in individuals exposed to arsenic through drinking water in West Bengal, India. Mutat Res 516:29–40
Basu A, Ghosh P, Das JK, Banerjee A, Ray K, Giri AK (2004) Micronuclei as biomarkers of carcinogen exposure in populations exposed to arsenic through drinking water in West Bengal, India: a comparative study in three cell types. Cancer Epidemiol Biomarkers Prev 13:820–827
Benbrahim-Tallaa L, Waterland RA, Styblo M, Achanzar WE, Webber MM, Waalkes MP (2005) Molecular events associated with arsenic-induced malignant transformation of human prostatic epithelial cells: aberrant genomic DNA methylation and K-ras oncogene activation. Toxicol Appl Pharmacol 106:288–298
Bhattacharjee P, Banerjee M, Giri AK (2013) Role of genomic instability in arsenic-induced carcinogenicity. A review. Environ Int 53:29–40. doi:10.1016/j.envint.2012.12.004
Biggs ML, Kalman DA, Moore LE, Hopenhayn-Rich C, Smith MT, Smith AH (1997) Relationship of urinary arsenic to intake estimates and a biomarker of effect, bladder cell micronuclei. Mutat Res 386:185–195
Bjornsson HT, Fallin MD, Feinberg AP (2004) An integrated epigenetic and genetic approach to common human disease. Trends Genet 20:350–358
Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, Holland N, Kirsch-Volders M, Zeiger E, Ban S, Barale R, Bigatti MP, Bolognesi C, Cebulska-Wasilewska A, Fabianova E, Fucic A, Hagmar L, Joksic G, Martelli A, Migliore L, Mirkova E, Scarfi MR, Zijno A, Norppa H, Fenech M (2007) An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis 28:625–631
Bonassi S, Norppa H, Ceppi M, Strömberg U, Vermeulen R, Znaor A, Cebulska-Wasilewska A, Fabianova E, Fucic A, Gundy S, Hansteen IL, Knudsen LE, Lazutka J, Rossner P, Sram RJ, Boffetta P (2008) Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: results from a pooled cohort study of 22358 subjects in 11 countries. Carcinogenesis 29:1178–1183. doi:10.1093/carcin/bgn075
Bun-ya M, Shikata K, Nakade S, Yompakdee C, Harashima S, Oshima Y (1996) Two new genes, PHO86 and PHO87, involved in inorganic phosphate uptake in Saccharomyces cerevisiae. Curr Genet 29:344–351. doi:10.1007/s002940050055
Cao Y, Yu SL, Wang Y, Guo GY, Ding Q, An RH (2011) MicroRNA-dependent regulation of PTEN after arsenic trioxide treatment in bladder cancer cell line T24. Tumour Biol 32:179–188. doi:10.1007/s13277-010-0111-z
Catanzaro I, Schiera G, Sciandrello G, Barbata G, Caradonna F, Proia P, Di Liegro I (2010) Biological effects of inorganic arsenic on primary cultures of rat astrocytes. Int J Mol Med 26:457–462
Caudill MA, Wang JC, Melnyk S, Pogribny IP, Jernigan S, Collins MD, Santos-Guzman J, Swendseid ME, Cogger EA, James SJ (2001) Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J Nutr 131:2811–2818
Chai CY, Huang YC, Hung WC, Kang WY, Chen WT (2007) Arsenic salt-induced DNA damage and expression of mutant p53 and COX-2 proteins in SV-40 immortalized human uroepithelial cells. Mutagenesis 22:403–408
Chakraborty T, Das U, Poddar S, Sengupta B, De M (2006) Micronuclei and chromosomal aberrations as biomarkers: a study in an arsenic exposed population in West Bengal, India. Bull Environ Contam Toxicol 76:970–976
Chanda S, Dasgupta UB, Guhamazumder D, Gupta M, Chaudhuri U, Lahiri S, Das S, Ghosh N, Chatterjee D (2006) DNA hypermethylation of promoter of gene p53 and p16 in arsenic-exposed people with and without malignancy. Toxicol Sci 89:431–437
Chen H, Liu J, Zhao CQ, Diwan BA, Merrick BA, Waalkes MP (2001) Association of c-myc overexpression and hyperproliferation with arsenite-induced malignant transformation. Toxicol Appl Pharmacol 175:260–268
Chen H, Liu J, Diwan BA, Barrett CJ, Waalkes MP (2004) Chronic inorganic arsenic exposure induces hepatic global and individual gene hypomethylation: implications for arsenic hepatocarcinogenesis. Carcinogenesis 25:1779–1786
Chen WT, Hung WC, Kang WY, Huang YC, Chau CY (2007) Urothelial carcinomas arising in arsenic-contaminated areas are associated with hypermethylation of the gene promoter of the death-associated protein kinase. Histopathology 51:785–792
Chiou HY, Chiou ST, Hsu TH, Chou YL, Tseng CH, Wei ML, Chen CJ (2001) Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8102 residents in an arseniasis-endemic area in northeastern Taiwan. Am J Epidemiol 153:411–418
Chowdhury UK, Biswas BK, Roy Chowdhury T, Samanta G, Mandal BK, Basu GC, Chanda CR, Lodh D, Saha KC, Mukherjee SK, Roy S, Kabir S, Quamruzzaman Q, Chakraborti D (2000a) Groundwater arsenic-contamination in Bangladesh and West Bengal, India. Environ Health Perspect 108:393–397
Chowdhury UK, Biswas BK, Roy Chowdhury T, Samanta G, Mandal BK, Basu GC, Mukherjee SC, Saha KC, Chakraborti D (2000b) Arsenic groundwater contamination and sufferings of people in West Bengal-India and Bangladesh. In: Roussel AM, Anderson RA, Favrier AE (eds) Trace elements in man and animals. Kluwer Academic/Plneum, New York, pp 645–650
Colognato R, Coppedè F, Ponti J, Sabbioni E, Migliore L (2007) Genotoxicity induced by arsenic compounds in peripheral human lymphocytes analysed by cytokinesis-block micronucleus assay. Mutagenesis 22:255–261
Coppedè F (2010) One-carbon metabolism and Alzheimer’s disease: focus on epigenetics. Curr Genomics 11:246–260. doi:10.2174/138920210791233090
Coppin JF, Qu W, Waalkes MP (2008) Interplay between cellular methyl metabolism and adaptive efflux during oncogenic transformation from chronic arsenic exposure in human cells. J Biol Chem 283:19342–19350. doi:10.1074/jbc.M802942200
Cress WD, Seto E (2000) Histone deacetylases, transcriptional control, and cancer. J Cell Physiol 184:1–16
Cui X, Wakai T, Shirai Y, Yokoyama N, Hatakeyama K, Hirano S (2006a) Arsenic trioxide inhibits DNA methyltransferase and restores methylation-silenced genes in human liver cancer cells. Hum Pathol 37:298–311
Cui X, Wakai T, Shirai Y, Hatakeyama K, Hirano S (2006b) Chronic oral exposure to inorganic arsenate interferes with methylation status of p16INK4a and RASSF1A and induces lung cancer in A/J mice. Toxicol Sci 91:372–381
Cui X, Kobayashi Y, Akashi M, Okayasu R (2008) Metabolism and the paradoxical effects of arsenic: carcinogenesis and anticancer. Curr Med Chem 15:2293–2304. doi:10.2174/092986708785747526
Cui Y, Han Z, Hu Y, Song G, Hao C, Xia H, Ma X (2012) MicroRNA-181b and microRNA-9 mediate arsenic-induced angiogenesis via NRP1. J Cell Physiol 227:772–783. doi:10.1002/jcp.22789
Das T, Roychoudhury A, Sharma A, Talukder G (1993) Modification of clastogenicity of three known clastogens by garlic extract in mice in vivo. Environ Mol Mutagen 21:383–388
Dauphiné DC, Smith AH, Yuan Y, Balmes JR, Bates MN, Steinmaus C (2013) Case–control study of arsenic in drinking water and lung cancer in California and Nevada. Int J Environ Res Public Health 10:3310–3324. doi:10.3390/ijerph10083310
Davis CD, Uthus EO (2004) DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med 229:988–995
Davis CD, Uthus EO, Finley JW (2000) Dietary selenium and arsenic affect DNA methylation in vitro in Caco-2 cells and in vivo in rat liver and colon. J Nutr 130:2903–2909
Deknudt G, Léonard A, Arany J, Jenar-Du Buisson G, Delavignette E (1986) In vivo studies in male mice on the mutagenic effects of inorganic arsenic. Mutagenesis 1:33–34
Dopp E, Hartmann LM, von Recklinghausen U, Florea AM, Rabieh S, Zimmermann U, Shokouhi B, Yadav S, Hirner AV, Rettenmeier AW (2005) Forced uptake of trivalent and pentavalent methylated and inorganic arsenic and its cyto-/genotoxicity in fibroblasts and hepatoma cells. Toxicol Sci 87:46–56. doi:10.1093/toxsci/kfi218
Dopp E, von Recklinghausen U, Diaz-Bone R, Hirner AV, Rettenmeier AW (2010) Cellular uptake, subcellular distribution and toxicity of arsenic compounds in methylating and non-methylating cells. Environ Res 110:435–442. doi:10.1016/j.envres.2009.08.012
Dopp E, von Recklinghausen U, Hippler J, Diaz-Bone RA, Richard J, Zimmermann U, Rettenmeier AW, Hirner AV (2011) Toxicity of volatile methylated species of bismuth, arsenic, tin, and mercury in mammalian cells in vitro. J Toxicol 2011:503576. doi:10.1155/2011/503576
Du J, Zhou N, Liu H, Jiang F, Wang Y, Hu C, Qi H, Zhong C, Wang X, Li Z (2012) Arsenic induces functional re-expression of estrogen receptor α by demethylation of DNA in estrogen receptor-negative human breast cancer. PLoS One 7:e35957. doi:10.1371/journal.pone.0035957
Eguchi N, Kuroda K, Endo G (1997) Metabolites of arsenic induced tetraploids and mitotic arrest in cultured cells. Arch Environ Contam Toxicol 32:141–145
Engström KS, Hossain MB, Lauss M, Ahmed S, Raqib R, Vahter M, Broberg K (2013) Efficient arsenic metabolism—the AS3MT haplotype is associated with DNA methylation and expression of multiple genes around AS3MT. PLoS One 8:e53732. doi:10.1371/journal.pone.0053732
Esteller M (2002) CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene 21:5427–5440
Esteve PO, Chin HG, Pradhan S (2007) Molecular mechanisms of transactivation and doxorubicin-mediated repression of surviving gene in cancer cells. J Biol Chem 282:2615–2625
Ferreccio C, Gonzalez C, Milosavjlevic G, Marshall G, Sancha AM, Smith AH (2000) Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology 11:673–679
Ferreccio C, Smith AH, Durán V, Barlaro T, Benítez H, Valdés R, Aguirre JJ, Moore LE, Acevedo J, Vásquez MI, Pérez L, Yuan Y, Liaw J, Cantor KP, Steinmaus C (2013) Case–control study of arsenic in drinking water and kidney cancer in uniquely exposed Northern Chile. Am J Epidemiol 178:813–818. doi:10.1093/aje/kwt059
Flora SJ (1999) Arsenic-induced oxidative stress and its reversibility following combined administration of N-acetylcysteine and meso 2,3-dimercaptosuccinic acid in rats. Clin Exp Pharmacol Physiol 26:865–869
Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI (2008) Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett 266:6–11. doi:10.1016/j.canlet.2008.02.026
Galanis A, Karapetsas A, Sandaltzopoulos R (2009) Metal-induced carcinogenesis, oxidative stress and hypoxia signaling. Mutat Res 674:31–35. doi:10.1016/j.mrgentox.2008.10.008
Gebel TW (2001) Genotoxicity of arsenical compounds. Int J Hyg Environ Health 203:249–262
Gebel T, Christensen S, Dunkelberg H (1997) Comparative and environmental genotoxicity of antimony and arsenic. Anticancer Res 17:2603–2607
Ghosh P, Basu A, Mahata J, Basu S, Sengupta M, Das JK, Mukherjee A, Sarkar AK, Mondal L, Ray K, Giri AK (2006) Cytogenetic damage and genetic variants in the individuals susceptible to arsenic-induced cancer through drinking water. Int J Cancer 118:2470–2478
Ghosh P, Banerjee M, De Chaudhuri S, Das JK, Sarma N, Basu A, Giri AK (2007) Increased chromosome aberration frequencies in the Bowen’s patients compared to non-cancerous skin lesions individuals exposed to arsenic. Mutat Res 632:104–110
Glozak MA, Seto E (2007) Histone deacetylases and cancer. Oncogene 26:5420–5432
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditioning on adult health and disease. N Engl J Med 359:61–73. doi:10.1056/NEJMra0708473
Gomez-Caminero A, Howe P, Hughes M, Kenyon E, Lewis DR, Moore M (2001) Arsenic and arsenic compounds. Environmental Health Criteria, 224. United Nations Environment Programme, the International Labour Organization, and the World Health Organization
Guil S, Esteller M (2009) DNA methylomes, histone codes and miRNAs: tying it all together. Int J Biochem Cell Biol 41:87–95. doi:10.1016/j.biocel.2008.09.005
Guillamet E, Creus A, Ponti J, Sabbioni E, Fortaner S, Marcos R (2004) In vitro DNA damage by arsenic compounds in a human lymphoblastoid cell line (TK6) assessed by the alkaline Comet assay. Mutagenesis 19:129–135
Hall A (2002) Chronic arsenic poisoning. Toxicol Lett 128:69–72
Han ZJ, Song G, Cui Y, Xia HF, Ma X (2011) Oxidative stress is implicated in arsenic-induced neural tube defects in chick embryos. Int J Dev Neurosci 29:673–680. doi:10.1016/j.ijdevneu.2011.06.006
Hasgekar N, Beck JP, Dunkelberg H, Hirsch-Ernst KI, Gebel WT (2006) Influence of antimonite, selenite, and mercury on the toxicity of arsenite in primary rat hepatocytes. Biol Trace Elem Res 111:167–183
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531
Heit R, Rattner JB, Chan GK, Hendzel MJ (2009) G2 histone methylation is required for the proper segregation of chromosomes. J Cell Sci 122:2957–2968. doi:10.1242/jcs.045351
Helleday T, Nilsson R, Jenssen D (2000) Arsenic[III] and heavy metal ions induce intrachromosomal homologous recombination in the hprt gene of V79 Chinese hamster cells. Environ Mol Mutagen 35:114–122
Hernández A, Sampayo-Reyes A, Marcos R (2011) Identification of differentially expressed genes in the livers of chronically i-As-treated hamsters. Mutat Res 713:48–55. doi:10.1016/j.mrfmmm.2011.05.013
Hinwood AL, Jolley DJ, Sim MR (1999) Cancer incidence and high environmental arsenic concentrations in rural populations: results of an ecological study. Int J Environ Health Res 9:131–141
Hitchler MJ, Domann FE (2009) Metabolic defects provide a spark for the epigenetic switch in cancer. Free Radic Biol Med 47:115–127. doi:10.1016/j.freeradbiomed.2009.04.010
Hopenhayn-Rich C, Biggs ML, Fuchs A, Bergoglio R, Tello EE, Nicolli H, Smith AH (1996) Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology 7:117–124
Hossain MB, Vahter M, Concha G, Broberg K (2012) Environmental arsenic exposure and DNA methylation of the tumor suppressor gene p16 and the DNA repair gene MLH1: effect of arsenic metabolism and genotype. Metallomics 4:1167–1175. doi:10.1039/c2mt20120h
Hsueh YM, Wu WL, Huang YL, Chiou HY, Tseng CH, Chen CJ (1998) Low serum carotene level and increased risk of ischemic heart disease related to long-term arsenic exposure. Atherosclerosis 141:249–257. doi:10.1016/S0021-9150(98)00178-6
Huang SC, Lee TC (1998) Arsenite inhibits mitotic division and perturbs spindle dynamics in HeLa S3 cells. Carcinogenesis 198:89–96. doi:10.1093/carcin/19.5.889
Huang C, Ke Q, Costa M, Shi X (2004) Molecular mechanisms of arsenic carcinogenesis. Mol Cell Biochem 255:57–66
Huff J, Waalkes M, Chan P (1998) Re: arsenic—evidence of carcinogenicity in animals. Environ Health Perspect 106:A582–A583
Huff J, Chan P, Nyska A (2000) Is the human carcinogen arsenic carcinogenic to laboratory animals? Toxicol Sci 55:17–23
Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133:1–16
Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ (2011) Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123:305–332. doi:10.1093/toxsci/kfr184
Intarasunanont P, Navasumrit P, Woraprasit S, Chaisatra K, Suk WA, Mahidol C, Ruchirawat M (2012) Effects of arsenic exposure on DNA methylation in cord blood samples from newborns babies and in a human lymphoblast cell line. Environ Health 11:31. doi:10.1186/1476-069X-11-31
International Agency for Research on Cancer (1980) Some metals and metallic compounds. IARC Monogr Eval Carcinog Risk Chem Hum 23:1–415
International Agency for Research on Cancer (1987) Overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl 7:1–440
International Agency for Research on Cancer (2004) Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risks Hum 84:1–477
International Agency for Research on Cancer (2012) Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum 100C:1–526
Jensen TJ, Novak P, Eblin KE, Gandolfi AJ, Futscher BW (2008) Epigenetic remodeling during arsenical-induced malignant transformation. Carcinogenesis 29:1500–1508. doi:10.1093/carcin/bgn102
Jensen TJ, Wozniak RJ, Eblin KE, Wnek SM, Gandolfi AJ, Futscher BW (2009) Epigenetic mediated transcriptional activation of WNT5A participates in arsenical-associated malignant transformation. Toxicol Appl Pharmacol 235:39–46. doi:10.1016/j.taap.2008.10.013
Jo WJ, Ren X, Chu F, Aleshin M, Wintz H, Burlingame A, Smith MT, Vulpe CD, Zhang L (2009) Acetylated H4K16 by MYST1 protects UROtsa cells from the carcinogen arsenic and is decreased following chronic arsenic exposure. Toxicol Appl Pharmacol 241:294–302. doi:10.1016/j.taap.2009.08.027
Kashiwada E, Kuroda K, Endo G (1998) Aneuploidy induced by dimethylarsinic acid in mouse bone marrow cells. Mutat Res 413:33–38
Kesari VP, Kumar A, Khan PK (2012) Genotoxic potential of arsenic at its reference dose. Ecotoxicol Environ Saf 80:126–131. doi:10.1016/j.ecoenv.2012.02.018
Khan PK, Kesari VP, Kumar A (2013) Mouse micronucleus assay as a surrogate to assess genotoxic potential of arsenic at its human reference dose. Chemosphere 90:993–997. doi:10.1016/j.chemosphere.2012.07.021
Kile ML, Baccarelli A, Hoffman E, Tarantini L, Quamruzzaman Q, Rahman M, Mahiuddin G, Mostofa G, Hsueh YM, Wright RO, Christiani DC (2012) Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environm Health Perspect 120:1061–1066. doi:10.1289/ehp.1104173
Kitchin KT (2001) Recent advances in arsenic and carcinogenesis modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol 172:249–261
Kitchin KT, Wallace K (2008) Evidence against the nuclear in situ binding of arsenicals–oxidative stress theory of arsenic carcinogenesis. Toxicol Appl Pharmacol 232:252–257. doi:10.1016/j.taap.2008.06.021
Klein CB, Leszczynska J, Hickey C, Rossman TG (2007) Further evidence against a direct genotoxic mode of action for arsenic-induced cancer. Toxicol Appl Pharmacol 222:289–297. doi:10.1016/j.taap.2006.12.033
Kligerman AD, Doerr CL, Tennant AH, Harrington-Brock K, Allen JW, Winkfield E, Poorman-Allen P, Kundu B, Funasaka K, Roop BC, Mass MJ, DeMarini DM (2003) Methylated trivalent arsenicals as candidate ultimate genotoxic forms of arsenic: induction of chromosomal mutations but not gene mutations. Environ Mol Mutagen 42:192–205. doi:10.1002/em.10192
Kligerman AD, Malik SI, Campbell JA (2010) Cytogenetic insights into DNA damage and repair of lesions induced by a monomethylated trivalent arsenical. Mutat Res 695:2–8. doi:10.1016/j.mrgentox.2009.09.007
Koestler DC, Avissar-Whiting M, Houseman EA, Karagas MR, Marsit C (2013) Differential DNA methylation in umbilical cord blood of infants exposed to low levels of arsenic in utero. Environ Health Perspect 121:971–977. doi:10.1289/ehp.1205925
Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705
Kuchino Y, Mori F, Kasai H, Inoue H, Iwai S, Miura K, Ohtsuka E, Nishimura S (1987) Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature 327:77–79
Kumar A, Kesari VP, Khan PK (2013) Fish micronucleus assay to assess genotoxic potential of arsenic at its guideline exposure in aquatic environment. Biometals 26:337–346. doi:10.1007/s10534-013-9620-8
Kuo TL (1968) Arsenic content of artesian well-water in endemic area of chronic arsenic poisoning. Rep Inst Pathol Natl Taiwan Univ 20:7–13
Kurttio P, Pukkala E, Kahelin A, Auvinen A, Pekkanen J (1999) Arsenic concentrations in well water and risk of bladder and kidney cancer in Finland. Environ Health Perspect 107:705–710
Lambrou A, Baccarelli A, Wright RO, Weisskopf M, Bollati V, Amarasiriwardena C, Vokonas P, Schwartz J (2012) Arsenic exposure and DNA methylation among elderly men. Epidemiology 23:668–676
Lamm SH, Robbins S, Zhou C, Lu J, Chen R, Feinleib M (2013) Bladder/lung cancer mortality in Blackfoot-disease (BFD)-endemic area villages with low (<150 μg/L) well water arsenic levels—an exploration of the dose–response Poisson analysis. Regul Toxicol Pharmacol 65:147–156. doi:10.1016/j.yrtph.2012.10.012
Lee TC, Oshimura M, Barrett JC (1985) Comparison of arsenic-induced cell transformation, cytotoxicity, mutation and cytogenetic effects in Syrian hamster embryo cells in culture. Carcinogenesis 6:1421–1426
Lengauer C, Kinzler KW, Vogelstein B (1997) DNA methylation and genetic instability in colorectal cancer cells. Proc Natl Acad Sci USA 94:2545–2550
Lewińska D, Arkusz J, Stańczyk M, Palus J, Dziubałtowska E, Stepnik M (2007) Comparison of the effects of arsenic and cadmium on benzo(a)pyrene-induced micronuclei in mouse bone-marrow. Mutat Res 632:37–43
Li E (2002) Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 3:662–673
Li JH, Rossman TG (1989) Inhibition of DNA ligase activity by arsenite: a possible mechanism of its comutagenesis. Mol Toxicol 2:1–9
Li J, Chen P, Sinogeeva N, Gorospe M, Wersto RP, Chrest FJ, Barnes J, Liu Y (2002) Arsenic trioxide promotes histone H3 phosphoacetylation at the chromatin of CASPASE-10 in acute promyelocytic leukemia cells. J Biol Chem 277:49504–49510
Li J, Gorospe M, Barnes J, Liu Y (2003) Tumor promoter arsenite stimulates histone H3 phosphoacetylation of protoncogenes c-fos and c-jun chromatin in human diploid fibroblast. J Biol Chem 278:13183–13191
Li H, Engström K, Vahter M, Broberg K (2012) Arsenic exposure through drinking water is associated with longer telomeres in peripheral blood. Chem Res Toxicol 25:2333–2339. doi:10.1021/tx300222t
Lim SO, Gu JM, Kim MS, Kim HS, Park YN, Park CK, Cho JW, Park YM, Jung G (2008) Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology 135:2128–2140. doi:10.1053/j.gastro.2008.07.027
Lin HJ, Sung TI, Chen CY, Guo HR (2013) Arsenic levels in drinking water and mortality of liver cancer in Taiwan. J Hazard Mater. doi:10.1016/j.jhazmat.2012.12.049
Ling M, Li Y, Xu Y, Pang Y, Shen L, Jiang R, Zhao Y, Yang X, Zhang J, Zhou J, Wang X, Liu Q (2012) Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-κB in arsenite-induced cell transformation. Free Radic Biol Med 52:1508–1518. doi:10.1016/j.freeradbiomed.2012.02.020
Liou SH, Lung JC, Chen YH, Yang T, Hsieh LL, Chen CJ, Wu TN (1999) Increased chromosome-type chromosome aberration frequencies as biomarkers of cancer risk in a blackfoot endemic area. Cancer Res 59:1481–1484
Liou SH, Chen YH, Loh CH, Yang T, Wu TN, Chen CJ, Hsieh LL (2002) The association between frequencies of mitomycin C-induced sister chromatid exchange and cancer risk in arseniasis. Toxicol Lett 129:237–243
Liu SX, Athar M, Lippai I, Waldren C, Hei TK (2001) Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc Natl Acad Sci USA 98:1643–1648
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435:834–838
Mahata J, Basu A, Ghoshal S, Sarkar JN, Roy AK, Poddar G, Nandy AK, Banerjee A, Ray K, Natarajan AT, Nilsson R, Giri AK (2003) Chromosomal aberrations and sister chromatid exchanges in individuals exposed to arsenic through drinking water in West Bengal, India. Mutat Res 534:133–143
Mahata J, Chaki M, Ghosh P, Das LK, Baidya K, Ray K, Natarajan AT, Giri AK (2004a) Chromosomal aberrations in arsenic-exposed human populations: a review with special reference to a comprehensive study in West Bengal, India. Cytogenet Genome Res 104:359–364
Mahata J, Ghosh P, Sarkar JN, Ray K, Natarajan AT, Giri AK (2004b) Effect of sodium arsenite on peripheral lymphocytes in vitro: individual susceptibility among a population exposed to arsenic through the drinking water. Mutagenesis 19:223–229
Majumdar S, Chanda S, Ganguli B, Mazumder DN, Lahiri S, Dasgupta UB (2010) Arsenic exposure induces genomic hypermethylation. Environ Toxicol 25:315–318. doi:10.1002/tox.20497
Mäki-Paakkanen J, Kurttio P, Paldy A, Pekkanen J (1998) Association between the clastogenic effect in peripheral lymphocytes and human exposure to arsenic through drinking water. Environ Mol Mutagen 32:301–313
Marsit CJ, Eddy K, Kelsey KT (2006a) MicroRNA responses to cellular stress. Cancer Res 66:10843–10848
Marsit CJ, Karagas MR, Danaee H, Liu M, Andrew A, Schned A, Nelson HH, Kelsey KT (2006b) Carcinogen exposure and gene promoter hypermethylation in bladder cancer. Carcinogenesis 27:112–116
Martínez V, Creus A, Venegas W, Arroyo A, Beck JP, Gebel TW, Surrallés J, Marcos R (2004) Evaluation of micronucleus induction in a Chilean population environmentally exposed to arsenic. Mutat Res 564:65–74
Martínez V, Creus A, Venegas W, Arroyo A, Beck JP, Gebel TW, Surrallés J, Marcos R (2005) Micronuclei assessment in buccal cells of people environmentally exposed to arsenic in northern Chile. Toxicol Lett 155:319–327
Martínez VD, Vucic EA, Adonis M, Gil L, Lam WL (2011) Arsenic biotransformation as a cancer promoting factor by inducing DNA damage and disruption of repair mechanisms. Mol Biol Int 2011:718974. doi:10.4061/2011/718974
Mass MJ, Wang L (1997) Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for a mechanism of carcinogenesis. Mutat Res 386:263–277
Mass MJ, Tennant A, Roop BC, Cullen WR, Styblo M, Thomas DJ, Kligerman AD (2001) Methylated trivalent arsenic species are genotoxic. Chem Res Toxicol 14:355–361
Matschullat J (2000) Arsenic in the geosphere—a review. Sci Total Environ 249:297–312
McGarvey KM, Fahrner JA, Greene E, Martens J, Jenuwein T, Baylin SB (2006) Silenced tumor suppressor genes reactivated by DNA demethylation do not return to a fully euchromatic chromatin state. Cancer Res 66:3541–3549
Michels KB (2010) The promises and challenges of epigenetic epidemiology. Exp Gerontol 45:297–301. doi:10.1016/j.exger.2009.12.011
Migliore L, Coppedè F (2009) Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res 667:82–97. doi:10.1016/j.mrfmmm.2008.10.011
Moore LE, Warner ML, Smith AH, Kalman D, Smith MT (1996) Use of the fluorescent micronucleus assay to detect the genotoxic effects of radiation and arsenic exposure in exfoliated human epithelial cells. Environ Mol Mutagen 27:176–184
Moore LE, Smith AH, Hopenhayn-Rich C, Biggs ML, Kalman DA, Smith MT (1997) Micronuclei in exfoliated bladder cells among individuals chronically exposed to arsenic in drinking water. Cancer Epidemiol Biomarkers Prev 6:31–36
Mourón SA, Grillo CA, Dulout FN, Golijow CD (2006) Induction of DNA strand breaks, DNA-protein crosslinks and sister chromatid exchanges by arsenite in a human lung cell line. Toxicol In Vitro 20:279–285
Muñiz-Ortiz JG, Wallace KA, Leinisch F, Kadiiska MB, Mason RP, Kligerman AD (2013) Catalase has a key role in protecting cells from the genotoxic effects oh monomethylarsonous acid: a highly active metabolite of arsenic. Environ Mol Mutagen 54:317–326. doi:10.1002/em.21780
Naranmandura H, Suzuki N, Suzuki KT (2006) Trivalent arsenicals are bound to proteins during reductive methylation. Chem Res Toxicol 19:1010–1018. doi:10.1021/tx060053f
National Research Council (1999) Arsenic in drinking water. National Academy Press, Washington, pp 1–310
National Research Council (2001) Arsenic in drinking water, 2001 update. National Academy Press, Washington, pp 1–244
Németi B, Gregus Z (2004) Glutathione-dependent reduction of arsenate in human erythrocytes—a process independent of purine nucleoside phosphorylase. Toxicol Sci 82:419–428. doi:10.1093/toxsci/kfh301
Nordstrom DK (2002) Public health. Worldwide occurrences of arsenic in ground water. Science 296:2143–2145. doi:10.1126/science.1072375
Ochi T, Kita K, Suzuki T, Rumpler A, Goessler W, Francesconi KA (2007) Cytotoxic, genotoxic and cell-cycle disruptive effects of thio-dimethylarsinate in cultured human cells and the role of glutathione. Toxicol Appl Pharmacol 228:59–67. doi:10.1016/j.taap.2007.11.023
O’Hagan HM, Wang W, Sen S, Destefano Shields C, Lee SS, Zhang YW, Clements EG, Cai Y, Van Neste L, Easwaran H, Casero RA, Sears CL, Baylin SB (2011) Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell 20:606–619. doi:10.1016/j.ccr.2011.09.012
Okoji RS, Yu RC, Maronpot RR, Froines JR (2002) Sodium arsenite administration via drinking water increases genome-wide and Ha-ras DNA hypomethylation in methyl-deficient C57BL/6J mice. Carcinogenesis 23:777–785
Ostrosky-Wegman P, Gonsebatt ME, Montero R, Vega L, Barba H, Espinosa J, Palao A, Cortinas C, García-Vargas G, del Razo LM, Cebrían M (1991) Lymphocyte proliferation kinetics and genotoxic findings in a pilot study on individuals chronically exposed to arsenic in Mexico. Mutat Res 250:477–482
Oya-Ohta Y, Kaise T, Ochi T (1996) Induction of chromosomal aberrations in cultured human fibroblasts by inorganic and organic arsenic compounds and the different roles of glutathione in such induction. Mutat Res 357:123–129
Patchsung M, Boonla C, Amnattrakul P, Dissayabutra T, Mutirangura A, Tosukhowong P (2012) Long interspersed nuclear element-1 hypomethylation and oxidative stress: correlation and bladder cancer diagnostic potential. PLoS One 7:37009. doi:10.1371/journal.pone.0037009
Peterson CL, Laniel MA (2004) Histones and histone modifications. Curr Biol 14:R546–R551
Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D, Factor-Litvak P, Graziano JH, Gamble MV (2007) Genomic methylation of peripheral blood leukocyte DNA: influences of arsenic and folate in Bangladeshi adults. Am J Clin Nutr 86:1179–1186
Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D, Factor-Litvak P, Graziano JH, Gamble MV (2009) Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ Health Perspect 117:254–260. doi:10.1289/ehp.11872
Pilsner JR, Hall MN, Liu X, Ilievski V, Slavkovich V, Levy D, Factor-Litvak P, Yunus M, Rahman M, Graziano JH, Gamble MV (2012) Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLoS One 7:e37147. doi:10.1371/journal.pone.0037147
Raab A, Feldman J (2005) Arsenic speciation in hair extracts. Anal Bioanal Chem 381:332–338. doi:10.1007/s00216-004-2796-6
Ramírez OA, García FP (2005) Genotoxic damage in zebra fish (Danio rerio) by arsenic in waters from Zimapan, Hidalgo, Mexico. Mutagenesis 20:291–295
Ramírez P, Eastmond DA, Laclette JP, Ostrosky-Wegman P (1997) Disruption of microtubule assembly and spindle formation as a mechanism for the induction of aneuploid cells by sodium arsenite and vanadium pentoxide. Mutat Res 386:291–298. doi:10.1016/S1383-5742(97)00018-5
Ramírez T, Stopper H, Hock R, Herrera LA (2007) Prevention of aneuploidy by S-adenosyl-methionine in human cells treated with sodium arsenite. Mutat Res 617:16–22
Ramírez T, Brocher J, Stopper H, Hock R (2008) Sodium arsenite modulates histone acetylation, histone deacetylase activity and HMGN protein dynamic in human cells. Chromosoma 117:147–157
Raml R, Rumpler A, Goessler W, Vahter M, Li L, Ochi T, Francesconi KA (2007) Thio-dimethylarsinate is a common metabolite in urine samples from arsenic-exposed women in Bangladesh. Toxicol Appl Pharmacol 222:374–380
Rasmussen RE, Menzel DB (1997) Variation in arsenic-induced sister chromatid exchange in human lymphocytes and lymphoblastoid cell lines. Mutat Res 386:299–306
Reichard JF, Schnekenburger M, Puga A (2007) Long term low-dose arsenic exposure induces loss of DNA methylation. Biochem Biophys Res Commun 352:188–192
Rosen BP (2002) Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp Biochem Physiol A Mol Integr Physiol 133:689–693. doi:10.1016/S1095-6433(02)00201-5
Rossman TG (1981) Effect of metals on mutagenesis and DNA repair. Environ Health Perspect 40:189–195
Rossman TG, Uddin AN, Burns FJ, Bosland MC (2001) Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol Appl Pharmacol 176:64–71
Roy M, Sinha D, Mukherjee S, Paul S, Bhattacharya RK (2008) Protective effect of dietary phytochemicals against arsenite induced genotoxicity in mammalian V79 cells. Indian J Exp Biol 46:690–697
RoyChoudhury A, Das T, Sharma A, Talukder G (1996) Dietary garlic extract in modifying clastogenic effects of inorganic arsenic in mice: two-generation studies. Mutat Res 359:165–170
Sadikovic B, Al-Romaih K, Squire JA, Zielenska M (2008) Cause and consequences of genetic and epigenetic alterations in human cancer. Curr Genomics 9:394–408. doi:10.2174/138920208785699580
Salazar AM, Sordo M, Ostrosky-Wegman P (2009) Relationship between micronuclei formation and p53 induction. Mutat Res 672:124–128. doi:10.1016/j.mrgentox.2008.10.015
Saleha Banu B, Danadevi K, Jamil K, Ahuja YR, Visweswara Rao K, Ishaq M (2001) In vivo genotoxic effect of arsenic trioxide in mice using comet assay. Toxicology 162:171–177
Sampayo-Reyes A, Hernández A, El-Yamani N, López-Campos C, Mayet-Machado E, Rincón-Castañeda CB, Limones-Aguilar Mde L, López-Campos JE, de León MB, González-Hernández S, Hinojosa-Garza D, Marcos R (2010) Arsenic induces DNA damage in environmentally exposed Mexican children and adults. Influence of GSTO1 and AS3MT polymorphisms. Toxicol Sci 117:63–71. doi:10.1093/toxsci/kfq173
Sandoval J, Esteller M (2012) Cancer epigenomics: beyond genomics. Curr Opin Genet Dev 22:50–55. doi:10.1016/j.gde.2012.02.008
Schaumlöffel N, Gebel T (1998) Heterogeneity of the DNA damage provoked by antimony and arsenic. Mutagenesis 13:281–286
Schläwicke Engström K, Nermell B, Concha G, Strömberg U, Vahter M, Broberg K (2009) Arsenic metabolism is influenced by polymorphisms in genes involved in one-carbon metabolism and reduction reactions. Mutat Res 667:4–14. doi:10.1016/j.mrfmmm.2008.07.003
Sciandrello G, Caradonna F, Mauro M, Barbata G (2004) Arsenic-induced DNA hypomethylation affects chromosomal instability in mammalian cells. Carcinogenesis 25:413–417
Sharma M, Sharma S, Arora A, Kaul D (2013) Regulation of cellular Cyclin D1 gene by arsenic is mediated through miR-2909. Gene 522:60–64. doi:10.1016/j.gene.2013.03.058
Shia WJ, Pattenden SG, Workman JL (2006) Histone H4 lysine 16 acetylation breaks the genome’s silence. Genome Biol 7:217
Singh KP, DuMond JW Jr (2007) Genetic and epigenetic changes induced by chronic low dose exposure to arsenic of mouse testicular Leydig cells. Int J Oncol 30:253–260
Singh KP, Kumari R, Treas J, DuMond JW (2011) Chronic exposure to arsenic causes increased cell survival, DNA damage, and increased expression of mitochondrial transcription factor A (mtTFA) in human prostate epithelial cells. Chem Res Toxicol 24:340–349. doi:10.1021/tx1003112
Sinha D, Roy M (2011) Antagonistic role of tea against sodium arsenite-induced oxidative DNA damage and inhibition of DNA repair in Swiss albino mice. J Environ Pathol Toxicol Oncol 30:311–322
Sinha D, Roy S, Roy M (2010) Antioxidant potential of tea reduces arsenite induced oxidative stress in Swiss albino mice. Food Chem Toxicol 48:1032–1039. doi:10.1016/j.fct.2010.01.016
Smeester L, Rager JE, Bailey KA, Guan X, Smith N, Garcia-Vargas G, Del Razo LM, Drobna Z, Helkar H, Styblo M, Fry RC (2011) Epigenetic changes in individuals with arsenicosis. Chem Res Toxicol 24:165–167. doi:10.1021/tx1004419
Sordo M, Herrera LA, Ostrosky-Wegman P, Rojas E (2001) Cytotoxic and genotoxic effects of As, MMA, and DMA on leukocytes and stimulated human lymphocytes. Teratog Carcinog Mutagen 21:249–260
Sterner DE, Berger SL (2000) Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev 64:435–459
Stevens JJ, Graham B, Walker AM, Tchounwou PB, Rogers C (2010) The effects of arsenic trioxide on DNA synthesis and genotoxicity in human colon cancer cells. Int J Environ Res Public Health 7:2018–2032. doi:10.3390/ijerph7052018
Suzuki KT, Mandal BK, Ogra Y (2002) Speciation of arsenic in body fluids. Talanta 58:111–119. doi:10.1016/S0039-9140(02)00260-6
Takahashi M, Barrett JC, Tsutsui T (2002) Transformation by inorganic arsenic compounds of normal Syrian hamster embryo cells into a neoplastic state in which they become anchorage-independent and cause tumors in newborn hamsters. Int J Cancer 99:629–634
Tennant AH, Kligerman AD (2011) Superoxide dismutase protects cells from DNA damage induced by trivalent methylated arsenicals. Environ Mol Mutagen 52:238–243. doi:10.1002/em.20609
Thomas DJ, Styblo M, Lin S (2001) The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol 176:127–144
Tian D, Ma H, Feng Z, Xia Y, Le XC, Ni Z, Allen J, Collins B, Schreinemachers D, Mumford JL (2001) Analyses of micronuclei in exfoliated epithelial cells from individuals chronically exposed to arsenic via drinking water in inner Mongolia, China. J Toxicol Environ Health A 64:473–484
Tinwell H, Stephens SC, Ashby J (1991) Arsenite as the probable active species in the human carcinogenicity of arsenic: mouse micronucleus assays on Na and K arsenite, orpiment, and Fowler’s solution. Environ Health Perspect 95:205–210
Treas J, Tyagi T, Singh KP (2013) Chronic exposure to arsenic, estrogen and their combination causes increased growth and transformation in human prostate epithelial cells potentially by hypermethylation-mediated silencing of MLH1. Prostate 73:1660–1672. doi:10.1002/pros.22701
Tsang V, Fry RC, Niculescu MD, Rager JE, Saunders J, Paul DS, Zeisel SH, Waalkes MP, Styblo M, Drobná Z (2012) The epigenetic effects on high prenatal folate intake in male mouse fetuses exposed in utero to arsenic. Toxicol Appl Pharmacol 264:439–450. doi:10.1016/j.taap.2012.08.022
Tseng WP (1977) Effects and dose response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect 19:109–119
Tsuda T, Babazono A, Yamamoto E, Kurumatani N, Mino Y, Ogawa T, Kishi Y, Aoyama H (1995) Ingested arsenic and internal cancer: a historical cohort study followed for 33 years. Am J Epidemiol 141:198–209
Tuck-Muller CM, Narayan A, Tsien F, Smeets DF, Sawyer J, Fiala ES, Sohn OS, Ehrlich M (2000) DNA hypomethylation and unusual chromosome instability in cell lines from ICF syndrome patients. Cytogenet Cell Genet 89:121–128
United States Environmental Protection Agency (2001) National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring. Fed Regist 66:6976–7066
Uthus EO, Davis C (2005) Dietary arsenic affects dimethylhydrazine-induced aberrant crypt formation and hepatic global DNA methylation and DNA methyltransferase activity in rats. Biol Trace Elem Res 103(2):133–145
Valenzuela OL, Borja-Aburto VH, Garcia-Vargas GG, Cruz-Gonzalez MB, Garcia-Montalvo EA, Calderon-Aranda ES, Del Razo LM (2005) Urinary trivalent methylated arsenic species in a population chronically exposed to inorganic arsenic. Environ Health Perspect 113:250–254
Valinluck V, Tsai HH, Rogstad DK, Burdzy A, Bird A, Sowers LC (2004) Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2). Nucleic Acids Res 32:4100–4108
Waalkes MP, Liu J, Ward JM, Diwan BA (2004) Mechanisms underlying arsenic carcinogenesis: hypersensitivity of mice exposed to inorganic arsenic during gestation. Toxicology 198:31–38
Wang TS, Shu YF, Liu YC, Jan KY, Huang H (1997) Glutathione peroxidase and catalase modulate the genotoxicity of arsenite. Toxicology 121(3):229–237
Wang YC, Chaung RH, Tung LC (2004) Comparison of the cytotoxicity induced by different exposure to sodium arsenite in two fish cell lines. Aquat Toxicol 69:67–79
Wang A, Kligerman AD, Holladay SD, Wolf DC, Robertson JL (2009) Arsenate and dimethylarsinic acid in drinking water did not affect DNA damage repair in urinary bladder transitional cells or micronuclei in bone marrow. Environ Mol Mutagen 50:760–770. doi:10.1002/em.20496
Wang Z, Zhao Y, Smith E, Goodall GJ, Drew PA, Brabletz T, Yang C (2011) Reversal and prevention of arsenic-induced human bronchial epithelial cell malignant transformation by microRNA-200b. Toxicol Sci 121:110–122. doi:10.1093/toxsci/kfr029
Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Hussain I, Momotaj H, Graziano JH (2004) Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 112:1329–1333. doi:10.1289/ehp.6964
Waterland RA, Michels KB (2007) Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nut 27:363–388
Weitzman SA, Turk PW, Milkowski DH, Kozlowski K (1994) Free radical adducts induce alterations in DNA cytosine methylation. Proc Natl Acad Sci USA 91:1261–1264
Wilhelm CS, Kelsey KT, Butler R, Plaza S, Gagne L, ScotZens M, Andrew AS, Morris S, Nelson HH, Schned AR (2010) Implications of LINE1 methylation for bladder cancer risk in women. Clin Cancer Res 16:1682–1689. doi:10.1158/1078-0432.CCR-09-2983
Wilhelm-Benartzi CS, Koestler DC, Kouseman EA, Christensen BC, Wiencke JK, Scned AR, Karagas MR, Kelsey KT, Marsit CJ (2010) DNA methylation profiles delineate etiologic heterogeneity and clinically important subgroups of bladder cancer. Carcinogenesis 31:1972–1976. doi:10.1093/carcin/bgq178
Xie Y, Trouba KJ, Liu J, Waalkes MP, Germolec DR (2004) Biokinetics and subchronic toxic effects of oral arsenite, monomethylarsonic acid, and dimethylarsinic acid in v-Ha-ras transgenic (Tg.AC) mice. Environ Health Perspect 112:1255–1263
Xie Y, Liu J, Benbrahim-Tallaa L, Ward JM, Logsdon D, Diwan BA, Waalkes MP (2007) Aberrant DNA methylation and gene expression in livers of newborn mice trnasplacentally exposed to a hepatocarcinogenic dose of inorganic arsenic. Toxicology 236:7–15
Yamanaka K, Okada S (1994) Induction of lung-specific DNA damage by metabolically methylated arsenics via the production of free radicals. Environ Health Perspect 102:37–40
Yamanaka K, Hasegawa A, Sawamura R, Okada S (1989) Dimethylated arsenics induce DNA strand breaks in lung via the production of active oxygen in mice. Biochem Biophys Res Commun 165:43–50
Yamanaka K, Kato K, Mizoi M, An Y, Takabayashi F, Nakano M, Hoshino M, Okada S (2004) The role of active arsenic species produced by metabolic reduction of dimethylarsinic acid in genotoxicity and tumorigenesis. Toxicol Appl Pharmacol 198:385–393
Yang HC, Fu HL, Lin YF, Rosen BP (2012) Pathways of arsenic uptake and efflux. Curr Top Membr 69:325–358. doi:10.1016/B978-0-12-394390-3.00012-4
Yedjou CG, Tchounwou PB (2007) In-vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell gel electrophoresis (Comet) assays. Mol Cell Biochem 301:123–130
Yedjou C, Sutton L, Tchounwou P (2008) Genotoxic mechanisms of arsenic trioxide in human Jurkat T-lymphoma cells. Met Ions Biol Med 10:495–499
You JS, Jones PA (2012) Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell 22:9–20. doi:10.1016/j.ccr.2012.06.008
Zawia NH, Lahiri DK, Cardozo-Pelaez F (2009) Epigenetics, oxidative stress, and Alzheimer disease. Free Radic Biol Med 46:1241–1249. doi:10.1016/j.freeradbiomed.2009.02.006
Zelko IN, Stepp MW, Vorst AL, Folz RJ (2011) Histone acetylation regulates the cell-specific and interferon-γ-inducible expression of extracellular superoxide dismutase in human pulmonary arteries. Am J Respir Cell Mol Biol 45:953–961. doi:10.1165/rcmb.2011-0012OC
Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP (1997) Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci USA 94:10907–10912
Zhou X, Sun H, Ellen TP, Chen H, Costa M (2008) Arsenite alters global histone H3 methylation. Carcinogenesis 29:1831–1836
Zhou X, Li Q, Arita A, Sun H, Costa M (2009) Effects of nickel, chromate, and arsenite on histone 3 lysine methylation. Toxicol Appl Pharmacol 236:78–84. doi:10.1016/j.taap.2009.01.009
Ziech D, Franco R, Pappa A, Panayiotidis MI (2011) Reactive oxygen species (ROS)-induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res 711:167–173. doi:10.1016/j.mrfmmm.2011.02.015
Acknowledgments
Elisa Bustaffa and Fabrizio Bianchi thank the Project “Sorveglianza epidemiologica in aree interessate da inquinamento ambientale da arsenico di origine naturale o antropica”, SEpiAs—CCM 2010, funded by the Italian Ministry of Health.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Elisa Bustaffa and Andrea Stoccoro have contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Bustaffa, E., Stoccoro, A., Bianchi, F. et al. Genotoxic and epigenetic mechanisms in arsenic carcinogenicity. Arch Toxicol 88, 1043–1067 (2014). https://doi.org/10.1007/s00204-014-1233-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1233-7