Abstract
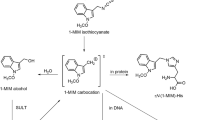
The common polycyclic aromatic hydrocarbon 1-methylpyrene is hepatocarcinogenic in the newborn mouse assay. In vitro studies showed that it is metabolically activated via benzylic hydroxylation and sulphation to a reactive ester, which forms benzylic DNA adducts, N 2-(1-methylpyrenyl)-2′-deoxyguanosine (MPdG) and N 6-(1-methylpyrenyl)-2′-deoxyadenosine (MPdA). Formation of these adducts was also observed in animals treated with the metabolites, 1-hydroxymethylpyrene and 1-sulphooxymethylpyrene (1-SMP), whereas corresponding data are missing for 1-methylpyrene. In the present study, we treated mice with 1-methylpyrene and subsequently analysed blood serum for the presence of the reactive metabolite 1-SMP and tissue DNA for the presence of MPdG and MPdA adducts. We used wild-type mice and a mouse line transgenic for human sulphotransferases (SULT) 1A1 and 1A2, males and females. All analyses were conducted using ultra-performance liquid chromatography coupled with tandem mass spectrometry, for the adducts with isotope-labelled internal standards. 1-SMP was detected in all treated animals. Its serum level was higher in transgenic mice than in the wild-type (p < 0.001). Likewise, both adducts were detected in liver, kidney and lung DNA of all exposed animals. The transgene significantly enhanced the level of each adduct in each tissue of both sexes (p < 0.01–0.001). Adduct levels were highest in the liver, the target tissue of carcinogenesis, in each animal model used. MPdG and MPdA adducts were also observed in rats treated with 1-methylpyrene. Our findings corroborate the hypothesis that 1-SMP is indeed the ultimate carcinogen of 1-methylpyrene and that human SULT are able to mediate the terminal activation in vivo.


Similar content being viewed by others
Abbreviations
- DMSO:
-
Dimethylsulphoxide
- dN:
-
2′-Deoxynucleoside(s)
- MAK:
-
Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (Germany)
- MPdA:
-
N 6-(1-methylpyrenyl)-2′-deoxyadenosine
- MPdG:
-
N 2-(1-methylpyrenyl)-2′-deoxyguanosine
- MRM:
-
Multiple reaction monitoring
- 1-SMP:
-
1-Sulphooxymethylpyrene
- SULT:
-
Sulphotransferase
- tg:
-
Hemizygous transgenic FVB/N mice carrying the human SULT1A1-SULT1A2 gene cluster in chromosome 9
- UPLC–MS/MS:
-
Ultra-performance liquid chromatography coupled with tandem mass spectrometry
- wt:
-
Wild-type FVB/N mice
References
Alnouti Y, Klaassen CD (2006) Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci 93:242–255
Bakhiya N, Stephani M, Bahn A, Ugele B, Seidel A, Burckhardt G, Glatt HR (2006) Uptake of chemically reactive, DNA-damaging sulfuric acid esters into renal cells by human organic anion transporters. J Am Soc Nephrol 17:1414–1421
Blumer M, Youngblood WW (1975) Polycyclic aromatic hydrocarbons in soils and recent sediments. Science 188:53–55
Conney AH (1982) Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res 42:4875–4917
Dobbernack G, Meinl W, Schade N, Florian S, Wend K, Voigt I, Himmelbauer H, Gross M, Liehr T, Glatt HR (2011) Altered tissue distribution of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-DNA adducts in mice transgenic for human sulfotransferases 1A1 and 1A2. Carcinogenesis 32:1734–1740
Enders N, Seidel A, Monnerjahn S, Glatt HR (1993) Synthesis of 11 benzylic sulfate esters, their bacterial mutagenicity and its modulation by chloride, bromide and acetate anions. Polycycl Aromat Compd 3:887s–894s
Engst W, Landsiedel R, Hermersdörfer H, Doehmer J, Glatt HR (1999) Benzylic hydroxylation of 1-methylpyrene and 1-ethylpyrene by human and rat cytochromes P450 individually expressed in V79 Chinese hamster cells. Carcinogenesis 20:1777–1785
Glatt HR (2000) Sulfotransferases in the bioactivation of xenobiotics. Chem Biol Interact 129:141–170
Glatt HR, Meinl W (2005) Sulfotransferases and acetyltransferases in mutagenicity testing: technical aspects. Methods Enzymol 400:230–249
Glatt HR, Oesch F (1986) Structural and metabolic parameters governing the mutagenicity of polycyclic aromatic hydrocarbons. In: de Serres FJ (ed) Chemical mutagens: principles and methods for their detection. Plenum Press, New York, pp 73–127
Glatt HR, Henschler R, Phillips DH, Blake JW, Steinberg P, Seidel A, Oesch F (1990) Sulfotransferase-mediated chlorination of 1-hydroxymethylpyrene to a mutagen capable of penetrating indicator cells. Environ Health Perspect 88:43–48
Glatt HR, Seidel A, Harvey RG, Coughtrie MW (1994) Activation of benzylic alcohols to mutagens by human hepatic sulphotransferases. Mutagenesis 9:553–557
Glatt HR, Bartsch I, Christoph S, Coughtrie MW, Falany CN, Hagen M, Landsiedel R, Pabel U, Phillips DH, Seidel A, Yamazoe Y (1998) Sulfotransferase-mediated activation of mutagens studied using heterologous expression systems. Chem Biol Interact 109:195–219
Glatt HR, Meinl W, Kuhlow A, Ma L (2003) Metabolic formation, distribution and toxicological effects of reactive sulphuric acid esters. Nova Acta Leopold NF87 329:151–161
Grimmer G (1979) Prozesse, bei denen PAH entstehen. Luftqualitätskriterien für ausgewählte polyzyklische aromatische Kohlenwasserstoffe. Erich Schmidt-Verlag, Berlin, pp 54–76
Grimmer G, Brune H, Dettbarn G, Naujack KW, Mohr U, Wenzel-Hartung R (1988) Contribution of polycyclic aromatic compounds to the carcinogenicity of sidestream smoke of cigarettes evaluated by implantation into the lungs of rats. Cancer Lett 43:173–177
Guillen MD, Sopelana P (2004) Occurrence of polycyclic aromatic hydrocarbons in smoked cheese. J Dairy Sci 87:556–564
Gupta RC (1984) Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci USA 81:6943–6947
Hartwig A (ed) (2013) The MAK-collection for occupational health and safety. Part I: MAK value documentations. Wiley, Weinheim
Herrmann K, Schumacher F, Engst W, Appel KE, Klein K, Zanger UM, Glatt HR (2013) Abundance of DNA adducts of methyleugenol, a rodent hepatocarcinogen, in human liver samples. Carcinogenesis 34:1025–1030
Herrmann K, Engst W, Meinl W, Florian S, Cartus AT, Nolden T, Himmelbauer H, Glatt HR (2013b) Formation of hepatic DNA adducts by methyleugenol in mouse models: drastic decrease by Sult1a1 knockout and strong increase by transgenic human SULT1A1/2. Carcinogenesis. doi:10.1093/carcin/bgt408
Hopia A, Pyysalo H, Wicksträm K (1986) Margarines, butter and vegetable oils as sources of polycyclic aromatic hydrocarbons. J Am Oil Chem Soc 63:889–893
Horn J, Flesher JW, Lehner AF (1996) 1-Sulfooxymethylpyrene is an electrophilic mutagen and ultimate carcinogen of 1-methyl- and 1-hydroxymethylpyrene. Biochem Biophys Res Commun 228:105–109
Larsson BK, Sahlberg GP, Eriksson AT, Busk LA (1983) Polycyclic aromatic hydrocarbons in grilled food. J Agric Food Chem 31:867–873
Lee ML, Novotny M, Bartle KD (1976) Gas chromatography/mass spectrometric and nuclear magnetic resonance spectrometric studies of carcinogenic polynuclear aromatic hydrocarbons in tobacco and marijuana smoke condensates. Anal Chem 48:405–416
Lee ML, Bartle KD, Novotny MV (1981) Analytical chemistry of polycyclic aromatic compounds. Academic Press, New York
Ma L, Kuhlow A, Glatt HR (2003) Albumin strongly prolongs the lifetime of chemically reactive sulphuric acid esters and affects their biological activities in the rat. Nova Acta Leopold 329:265–272
Monien BH, Müller C, Engst W, Frank H, Seidel A, Glatt HR (2008) Time course of hepatic 1-methylpyrene DNA adducts in rats determined by isotope dilution LC–MS/MS and 32P-postlabeling. Chem Res Toxicol 21:2017–2025
Monien BH, Müller C, Bakhiya N, Donath C, Frank H, Seidel A, Glatt HR (2009) Probenecid, an inhibitor of transmembrane organic anion transporters, alters tissue distribution of DNA adducts in 1-hydroxymethylpyrene-treated rats. Toxicology 262:80–85
Monnerjahn S, Seidel A, Steinberg P, Oesch F, Hinz M, Stezowsky JJ, Hewer A, Phillips DH, Glatt HR (1993) Formation of DNA adducts from 1-hydroxymethylpyrene in liver cells in vivo and in vitro. In: Phillips DH, Castegnaro M, Bartsch H (eds) Postlabelling methods for detection of DNA adducts. IARC, Lyon, pp 189–193
Okamoto H, Yoshida DR (1981) Metabolic formation of pyrenequinones as enhancing agents of mutagenicity in Salmonella. Cancer Lett 11:215–220
Orecchio S, Ciotti VP, Culotta L (2009) Polycyclic aromatic hydrocarbons (PAHs) in coffee brew samples: analytical method by GC–MS, profile, levels and sources. Food Chem Toxicol 47:819–826
Pancirov RJ, Brown RA (1977) Polynuclear aromatic hydrocarbons in marine tissues. Environ Sci Technol 11:989–992
Radke M (1987) Organic geochemistry of aromatic hydrocarbons. In: Brooks J, Welte DH (eds) Advances in petroleum geochemistry. Academic Press, London, pp 141–207
Renaud H, Cui J, Khan M, Klaassen C (2011) Tissue distribution and gender-divergent expression of 78 cytochrome P450 mRNAs in mice. Toxicol Sci 124:261–277
Rice JE, Rivenson A, Braley J, LaVoie EJ (1987) Methylated derivatives of pyrene and fluorene: evaluation of genotoxicity in the hepatocyte/DNA repair test and tumorigenic activity in newborn mice. J Toxicol Environ Health 21:525–532
Rice JE, Geddie NG, DeFloria MC, LaVoie EJ (1988) Structural requirements favoring mutagenic activity among methylated pyrenes in S. typhimurium. In: Cooke M, Dennis A (eds) Polynuclear aromatic hydrocarbons: a decade of progress. Battelle Press, Columbus, OH, pp 773–785
Rodgman A, Perfetti TA (2006) The composition of cigarette smoke: a catalogue of polycyclic aromatic hydrocarbons. Beitr zur Tabakforsch Int 22:13–69
Ruvinsky A, Marshall Graves JA (eds) (2005) Mammalian genomics. CABI Publishing, Wallingford, Oxfordshire
Schenck PA, de Leeuw JW (1982) Molecular organic geochemistry. In: Hutzinger O (ed) Handbook of environmental chemistry. Springer, Berlin, pp 111–129
Seidel A, Glatt HR, Oesch F, Garrigues P (1990) 2,6-Dimethylpicene: synthesis, mutagenic activity, and identification in natural samples. Polycycl Aromat Compd 1:3–14
Severson RF, Snook ME, Arrendale RF, Chortyk OT (1976) Gas chromatographic quantitation of polynuclear aromatic hydrocarbons in tobacco smoke. Anal Chem 48:1866–1872
Snook ME, Severson RF, Arrendale RF, Higman HC, Chortyk OT (1978) Multi-alkylated polynuclear aromatic hydrocarbons of tobacco smoke: separation and identification. Beitr Tabakforsch Int 9:222–247
Surh YJ, Blomquist JC, Liem A, Miller JA (1990) Metabolic activation of 9-hydroxymethyl-10-methylanthracene and 1-hydroxymethylpyrene to electrophilic, mutagenic and tumorigenic sulfuric acid esters by rat hepatic sulfotransferase activity. Carcinogenesis 11:1451–1460
Thakker DR, Yagi H, Levin W, Wood AW, Conney AH, Jerina DM (1985) Polycyclic aromatic hydrocarbons: metabolic activation to ultimate carcinogens. In: Anders MW (ed) Bioactivation of foreign compounds. Academic Press, Inc., Orlando, FL, pp 177–242
Tretyakova NY, Goggin M, Sangaraju D, Janis G (2012) Quantitation of DNA adducts by stable isotope dilution mass spectrometry. Chem Res Toxicol 25:2007–2035
Watabe T, Ishizuka T, Isobe M, Ozawa N (1982) A 7-hydroxymethyl sulfate ester as an active metabolite of 7,12-dimethylbenz[a]anthracene. Science 215:403–405
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bendadani, C., Meinl, W., Monien, B.H. et al. The carcinogen 1-methylpyrene forms benzylic DNA adducts in mouse and rat tissues in vivo via a reactive sulphuric acid ester. Arch Toxicol 88, 815–821 (2014). https://doi.org/10.1007/s00204-013-1182-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1182-6