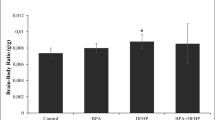
Abstract
Di(2-ethylhexyl)phthalate (DEHP) induced adverse effects on mice offspring, and the metabolite mono(2-ethylhexyl)phthalate (MEHP) may be essential to determine the toxicity. In this experiment, we measured liver MEHP levels and the factors determining the metabolism, two enzyme activities [lipase and uridine 5′-diphosphate-glucuronosyltransferase (UGT)] or expression of cytochrome P450 4A14 (CYP4A14) in dams (on gestational day 18 and postnatal day 2) and their offspring. MEHP concentrations in the liver from pregnant dams were 1.5 times higher than those of postpartum dams at exposure to 0.05% DEHP. Accordingly, MEHP concentrations were 1.7 times higher in fetuses than in pups at the dose. Interestingly, lipase activity was 1.8-fold higher in pregnant dams than postpartum ones, but no such difference was noted in the activity between fetuses and pups. UGT activity was also 1.5-fold higher in pregnant dams than postpartum ones, whereas the activity in the fetuses was 1/2 that of pups. No difference was noted in CYP4A14 levels between pregnant and postpartum mice, whereas the levels in the fetuses were <1/10 those of pups. DEHP exposure did not influence lipase activity, whereas it slightly enhanced UGT activity and exclusively increased CYP4A14 levels in pregnant and/or postpartum dams. Taken together, the higher MEHP levels in pregnant dams than postpartum ones may be primarily due to higher lipase activities in pregnant dams, which may closely reflect those in fetuses and pups.





Similar content being viewed by others
References
Albro PW, Lavenhar SR (1989) Metabolism of di(2-ethylhexyl)phthalate. Drug Metab Rev 21:13–34
Anderson GD (2005) Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44:989–1008
Dostal LA, Weaver RP, Schwetz BA (1987) Transfer of di(2-ethylhexyl)phthalate through rat milk and effects on milk composition and the mammary gland. Toxicol Appl Pharmacol 91:315–325
Gollamudi R, Rao RH, Lawrence WH, Autian J (1985) Developmental changes in the conversion rates of di(2-ethylhexyl)phthalate to monoethylhexyl phthalate in rats. J Toxicol Environ Health 15:459–465
Hayashi Y, Ito Y, Yamagishi N, Yanagiba Y, Tamada H, Wang D, Ramdhan DH, Naito H, Harada Y, Kamijima M, Gonzales FJ, Nakajima T (2011) Hepatic peroxisome proliferator-activated receptor alpha may have an important role in the toxic effects of di(2-ethylhexyl)phthalate on offspring of mice. Toxicology 289:1–10
He XJ, Ejiri N, Nakayama H, Doi K (2005) Effects of pregnancy on CYPs protein expression in rat liver. Exp Mol Pathol 78:64–70
Ito Y, Yokota H, Wang R, Yamanoshita O, Ichihara G, Wang H, Kurata Y, Takagi K, Nakajima T (2005) Species differences in the metabolism of di(2-ethylhexyl)phthalate (DEHP) in several organs of mice, rats, and marmosets. Arch Toxicol 79:147–154
Ito Y, Yamanoshita O, Kurata Y, Kamijima M, Aoyama T, Nakajima T (2007) Induction of peroxisome proliferator-activated receptor alpha (PPARalpha)-related enzymes by di(2-ethylhexyl)phthalate (DEHP) treatment in mice and rats, but not marmosets. Arch Toxicol 81:219–226
Japan Plasticizer Industry Association (2010) Statistical data of production and shipment. http://www.kasozai.gr.jp/data/toukei-pdf/2011-05seisan.pdf. Accessed 14 Jul 2011
Kavlock R, Barr D, Boekelheide K, Breslin W, Breysse P, Chapin R, Gaido K, Hodgson E, Marcus M, Shea K, Williams P (2006) NTP-CERHR expert panel update on the reproductive and developmental toxicity of di(2-ethylhexyl)phthalate. Reprod Toxicol 22:291–399
Kishi M, Emi Y, Sakaguchi M, Ikushiro S, Iyanagi T (2008) Ontogenic isoform switching of UDP-glucuronosyltransferase family 1 in rat liver. Biochem Biophys Res Commun 377:815–819
Kuntzman R, Jacobson M, Schneidman K, Conney AH (1964) Similarities between oxidative drug-metabolizing enzymes and steroid hydroxylases in liver microsomes. J Pharmacol Exp Ther 146:280–285
Kuntzman R, Sansur M, Conney AH (1965) Effect of drugs and insecticides on the anesthetic action of steroids. Endocrinology 77:952–954
Lamb JCT, Chapin RE, Teague J, Lawton AD, Reel JR (1987) Reproductive effects of four phthalic acid esters in the mouse. Toxicol Appl Pharmacol 88:255–269
Lof M, Hilakivi-Clarke L, Sandin SS, de Assis S, Yu W, Weiderpass E (2009) Dietary fat intake and gestational weight gain in relation to estradiol and progesterone plasma levels during pregnancy: a longitudinal study in Swedish women. BMC Womens Health 9:10
Luquita MG, Catania VA, Pozzi EJ, Veggi LM, Hoffman T, Pellegrino JM, Ikushiro S, Emi Y, Iyanagi T, Vore M, Mottino AD (2001) Molecular basis of perinatal changes in UDP-glucuronosyltransferase activity in maternal rat liver. J Pharmacol Exp Ther 298:49–56
Maglich JM, Lobe DC, Moore JT (2009) The nuclear receptor CAR (NR1I3) regulates serum triglyceride levels under conditions of metabolic stress. J Lipid Res 50:439–445
Maloney EK, Waxman DJ (1999) Trans-activation of PPAR alpha and PPAR gamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol 161:209–218
Neale MG, Parke DV (1973) Effects of pregnancy on the metabolism of drugs in the rat and rabbit. Biochem Pharmacol 22:1451–1461
Pelkonen O (1980) Biotransformation of xenobiotics in the fetus. Pharmacol Ther 10:261–281
Ramdhan DH, Kamijima M, Yamada N, Ito Y, Yanagiba Y, Nakamura D, Okamura A, Ichihara G, Aoyama T, Gonzalez FJ, Nakajima T (2008) Molecular mechanism of trichloroethylene-induced hepatotoxicity mediated by CYP2E1. Toxicol Appl Pharmacol 231:300–307
Ramdhan DH, Ito Y, Yanagiba Y, Yamagishi N, Hayashi Y, Li C, Taneda S, Suzuki AK, Watanabe G, Taya K, Kamijima M, Nakajima T (2009) Nanoparticle-rich diesel exhaust may disrupt testosterone biosynthesis and metabolism via growth hormone. Toxicol Lett 191:103–108
Singh AR, Lawrence WH, Autian J (1975) Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. J Pharm Sci 64:1347–1350
Sjoberg P, Bondesson U, Gustafsson J (1988) Metabolism of mono-(2-ethylhexyl)phthalate in fetal, neonatal and adult rat liver. Biol Neonate 53:32–38
Suna S, Jitsunari F, Asakawa F, Kitamado T, Ohnishi S, Hirao T, Fukunaga I (2001) Simplified determination of di(2-ethylhexyl)phthalate (DEHP) and mono(2-ethylhexyl)phthalate (MEHP) in blood and organs of the rat administered DEHP. Sangyo Eiseigaku Zasshi 43:73–75 (in Japanese)
Suzuki Y, Niwa M, Yoshinaga J, Watanabe C, Mizumoto Y, Serizawa S, Shiraishi H (2009) Exposure assessment of phthalate esters in Japanese pregnant women by using urinary metabolite analysis. Environ Health Prev Med 14:180–187
Wang RS, Nakajima T, Honma T (1999) Trichloroethylene inhibits aldehyde dehydrogenase only for aliphatic aldehydes of short chains in rats. Toxicology 132:9–18
Wang Y, Botolin D, Christian B, Busik J, Xu J, Jump DB (2005) Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J Lipid Res 46:706–715
Yan X, Calafat A, Lashley S, Smulian J, Ananth C, Barr D, Silva M, Ledoux T, Hore P, Robson MG (2009) Phthalates biomarker identification and exposure estimates in a population of pregnant women. Hum Ecol Risk Assess 15:565–578
Yano T (1993) Sexing of in vitro-fertilized preimplantation mouse embryos by the PCR method. Jpn J Hum Genet 38:277–288
Acknowledgments
This work was supported by Grants-in-Aid from Food Safety Commission, Japan (No. 1002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yumi Hayashi and Yuki Ito contributed equally to this article.
Rights and permissions
About this article
Cite this article
Hayashi, Y., Ito, Y., Yanagiba, Y. et al. Differences in metabolite burden of di(2-ethylhexyl)phthalate in pregnant and postpartum dams and their offspring in relation to drug-metabolizing enzymes in mice. Arch Toxicol 86, 563–569 (2012). https://doi.org/10.1007/s00204-011-0790-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0790-2