Abstract
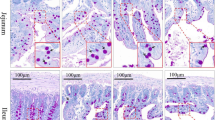
To investigate the effects of silver nanoparticles on the histological structure and properties of the mucosubstances in the intestinal mucosa, Sprague–Dawley rats were divided into four groups (10 rats in each group): vehicle control, low-dose group (30 mg/kg), middle-dose group (300 mg/kg), and high-dose group (1,000 mg/kg), and administered silver nanoparticles (60 nm) for 28 days, following OECD test guideline 407 and using GLP. The control sections contained no silver nanoparticles; however, the treated samples showed luminal and surface particles and the tissue also contained silver nanoparticles. A dose-dependent increased accumulation of silver nanoparticles was observed in the lamina propria in both the small and large intestine, and also in the tip of the upper villi in the ileum and protruding surface of the fold in the colon. The silver nanoparticle-treated rats exhibited higher numbers of goblet cells that had released their mucus granules than the controls, resulting in more mucus materials in the crypt lumen and ileal lumen. Moreover, cell shedding at the tip of the villi was frequent. Lower amounts of neutral and acidic mucins were found in the goblet cells in the silver nanoparticle-treated rats, plus the amount of sialomucins was increased, while the amount of sulfomucins was decreased. In particular, in the colon of the silver nanoparticle-treated rats, sialyated mucins were detected in the lamina propria, the connective tissue under the epithelia. Therefore, the present results suggest that silver nanoparticles induce the discharge of mucus granules and an abnormal mucus composition in the goblet cells in the intestines.





Similar content being viewed by others
References
Allen A, Pain RH, Robson TR (1976) Model for the structure of the gastric mucous gel. Nature 264:88–89
ATSDR (Agency for Toxic Substances and Disease Registry) (2005) Toxprofiles 2005, US Department of Health & Human Services, Atlanta, GA
Belly A, Keller K, Gottke M, Chadee K, Goettke M (1999) Intestinal mucins in colonization and host defense against pathogens. Am Trop Med Hyg 60:10–15
Blanchard C, Durual S, Estienne M, Bouzakri K, Heim MH, Blin N, Cuber JC (2004) IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J Immunol 172:3775–3783
Branka JE, Vallette G, Jarry A, Laboisse CL (1997) Stimulation of mucin exocytosis from human epithelial cells by nitric oxide: evidence for a cGMP-dependent and a cGMP-independent pathway. Biochem J 323:521–524
Dawson PA, Patel I, Filipe MI (1978) Variation in sialomucins in the mucosa of the large intestine in malignancy: a quantimet and statistical analysis. Histochem J 10:559–572
Feng QL, Cui FZ, Kim TN, Kim JW (1999) Ag-substituted hydroxyapatite coatings with both antimicrobial effects and biocompatibility. J Mater Sci Lett 18:559–561
Filipe MT (1971) The mucous membrane of the normal human large intestine and the changes which occur in it immediately adjacent to proven carcinoma—a histochemical, autoradiographic and chemical study. PhD thesis, University of London
Filipe MI, Branfoot AC (1974) Abnormal patterns of mucous secretion in apparently normal mucosa of large intestine with carcinoma. Cancer 34:282–290
Filipe MI, Cooke BK (1974) Changes in mucin composition in the mucosa adjacent to carcinoma of the colon as compared with the normal—a biochemical investigation. J Clin Pathol 27:315–318
Filipe MI, Dawson I (1970) The diagnostic value of mucosubstances in rectal biopsies from patients with ulcerative colitis and Crohn’s disease. Gut 11(3):229–234
Filipe MI, Fenger C (1979) Histochemical characteristics of mucins in the small intestine. A comparative study of normal mucosa, benign epithelial tumours and carcinoma. Histochem J 11(3):277–287
Florence AT, Hussain N (2001) Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv Drug Deliv Rev 50:S69–S89
Forstner J, Oliver M, Sylvester F (1995) Production, structure and biologic relevance of gastrointestinal mucins. In: Blaser M, Smith P, Ravdin J, Greenberg H, Guerrant R (eds) Infections of the gastrointestinal tract. Raven Press, New York, pp 71–88
Gupta A, Silver S (1998) Silver as biocide: will resistance become a problem? Nat Biotechnol 16:888
Hussain N, Jaitley V, Florence AT (2001) Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev 50:107–142
Ji JH, Jung JH, Kim SS, Yoon JU, Park JD, Choi BS, Chung YH, Kwon IH, Jeong J, Han BS, Shin JH, Sung JH, Song KS, Yu IJ (2007) A twenty-eight-days inhalation toxicity study of silver nanoparticles in Sprague–Dawley rats. Inhalat Toxicol 19(10):857–871
Jo UB, Kim BS (1987) Histochemical study on the effect of the pyridine herbicide, gramoxone, on the mucosubstances of the goblet cells in the rat small intestine. Korean J Anat 20(2):299–317 (in Korean)
Kemper AC, Specian RD (1991) Rat small intestinal mucins: a quantitative analysis. Anat Rec 229:219–226
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3:95–101
Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH, Kwon IH, Jeong J, Han BS, Yu IJ (2008) Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague–Dawley Rats. Inhalat Toxicol 20(6):575–583
KISTI (Korea Institute of Science and Technology Information) (2006) The present status of Korean nanotechnology industrialization. Nano Weekly, No. 210. KISTI, Seoul, Korea
Lee MK (1979) Histochemical studies on the effect of organophosphorus pesticides on the mucosubstances in the duodenal glands and goblet cells of the duodenal mucosa in the rat. Korean J Anat 12:111–126 (in Korean)
Li P, Li J, Wu C, Wu Q, Li J (2005) Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 16:1912–1917
Li Y, Leung P, Yao L, Song QW, Newton E (2006) Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect 62:58–63
Liau SY, Read DC, Pugh WJ, Furr JR, Russell AD (1997) Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett Appl Microbiol 25:279–283
Maynard AD (2006) Nanotechnology: a research strategy for addressing risk. Woodrow Wilson International Center for Scholars, Washington, DC
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Neutra MR, Forstner JF (1987) Gastrointestinal mucus: synthesis, secretion and function. In: Johnson LR (ed) Physiology of the gastrointestinal tract. Raven Press, New York, pp 975–1009
Neutra MR, O’Malley LJ, Specian RD (1982) Regulation of intestinal goblet cell secretion. A survey of potential secretagogues. Am J Physiol Gastrointest Liver Physiol 242:G380–G387
Nomiya K, Yoshizawa A, Tsukagoshi K, Kasuga NC, Hirakawa S, Watanabe J (2004) Synthesis and structural characterization of silver (I), aluminium (III) and cobalt (II) complexes with 4-isopropyltropolone (hinokitiol) showing noteworthy biological activities. Action of silver (I)-oxygen bonding complexes on the antimicrobial activities. J Inorg Biochem 98:46–60
OECD (1995) OECD guidelines for the testing of chemicals, Test guideline 407. Repeated dose 28-day oral toxicity study in rodent, Paris
Percival SL, Bowler PG, Russell D (2005) Bacterial resistance to silver in wound care. J Hosp Infect 60:1–7
Plaisancie P, Barcelo A, Moro F, Claustre J, Chayvialle JA, Cuber JC (1998) Effects of neurotransmitters, gut hormones, and inflammatory mediators on mucus discharge in rat colon. Am J Physiol Gastrointest Liver Physiol 275:G1073–G1084
Smirnova MG, Guo L, Birchall JP, Pearson JP (2003) LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol 221:42–49
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interf Sci 275:177–182
Verburg M, Renes IB, Van Nispen DJ, Ferdinandusse S, Jorritsma M, Buller HA, Einerhand AW, Dekker J (2002) Specific responses in rat small intestinal epithelial mRNA expression and protein levels during chemotherapeutic damage and regeneration. J Histochem Cytochem 50:1525–1536
Acknowledgments
This research was supported by Nano R&D program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2009-0082677).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, G.N., Jo, U.B., Ryu, H.Y. et al. Histochemical study of intestinal mucins after administration of silver nanoparticles in Sprague–Dawley rats. Arch Toxicol 84, 63–69 (2010). https://doi.org/10.1007/s00204-009-0469-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-009-0469-0