Abstract
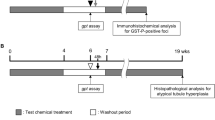
The objectives of this study were twofold: (1) evaluating the carcinogenic potential of the mixture of two persistent environmental pollutants, hexachlorobenzene (HCB) and 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126), in an initiation-promotion bioassay involving the development of π glutathione S-transferase (GST-P) liver foci, and (2) analyzing the GST-P foci data using a biologically-based computer model (i.e., clonal growth model) with an emphasis on the effect of focal size on the growth kinetics of initiated cells. The 8-week bioassay involved a series of treatments of initiator, two-thirds partial hepatectomy, and daily oral gavage of the mixture of two doses in male F344 rats. The mixture treatment significantly increased liver GST-P foci development, indicating carcinogenic potential of this mixture. Our clonal growth model was developed to simulate the appearance and development of initiated GST-P cells in the liver over time. In the model, the initiated cells were partitioned into two subpopulations with the same division rate but different death rates. Each subpopulation was further categorized into single cells, mini- (2–11 cells), medium- (12–399 cells), and large-foci (>399 cells) with different growth kinetics. Our modeling suggested that the growth of GST-P foci is size-dependent; in general, the larger the foci, the higher the rate constants of division and death. In addition, the modeling implied that the two doses promoted foci development in different manners even though the experimental foci data appeared to be similar between the two doses. This study further illustrated how clonal growth modeling may facilitate our understanding in chemical carcinogenic process.





Similar content being viewed by others
References
Bager Y, Hemming H, Flodstrom S, Ahlborg UG, Warngard L (1995) Interaction of 3,4,5,3′,4′-pentachlorobiphenyl and 2,4,5,2′,4′,5′-hexachlorobiphenyl in promotion of altered hepatic foci in rats. Pharmacol Toxicol 77:149–154
Bager Y, Kato Y, Kenne K, Warngard L (1997) The ability to alter the gap junction protein expression outside GST-P positive foci in liver of rats was associated to the tumour promotion potency of different polychlorinated biphenyls. Chem Biol Interact 103:199–212
Blais JM, Froese KL, Kimpe LE, Muir DC, Backus S, Comba M, Schindler DW (2003) Assessment and characterization of polychlorinated biphenyls near a hazardous waste incinerator: analysis of vegetation, snow, and sediments. Environ Toxicol Chem 22:126–133
Buchmann A, Stinchcombe S, Korner W, Hagenmaier H, Bock KW (1994) Effects of 2,3,7,8-tetrachloro- and 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin on the proliferation of preneoplastic liver cells in the rat. Carcinogenesis 15:1143–1150
Cabral R, Hoshiya T, Hakoi K, Hasegawa R, Ito N (1996) Medium-term bioassay for the hepatocarcinogenicity of hexachlorobenzene. Cancer Lett 100:223–226
Conolly RB, Andersen ME (1997) Hepatic foci in rats after diethylnitrosamine initiation and 2,3,7,8-tetrachlorodibenzo-p-dioxin promotion: evaluation of a quantitative two-cell model and of CYP 1A1/1A2 as a dosimeter. Toxicol Appl Pharmacol 146:281–293
Conolly RB, Kimbell JS (1994) Computer simulation of cell growth governed by stochastic processes: application to clonal growth cancer models. Toxicol Appl Pharmacol 124:284–295
Dean CE Jr, Benjamin SA, Chubb LS, Tessari JD, Keefe TJ (2002) Nonadditive hepatic tumor promoting effects by a mixture of two structurally different polychlorinated biphenyls in female rat livers. Toxicol Sci 66:54–61
Delesse M (1848) Procede mecanique pour determiner la composition des roches. Ann Mines 13:379–388
Dewanji A, Venzon DJ, Moolgavkar SH (1989) A stochastic two-stage model for cancer risk assessment. II. The number and size of premalignant clones. Risk Anal 9:179–187
Erturk E, Lambrecht RW, Peters HA, Cripps DJ, Gocmen A, Morris CR, Bryan GT (1986) Oncogenicity of hexachlorobenzene. IARC Sci Publ 77:417–423
Fukuhara Y, Hirasawa A, Li XK, Kawasaki M, Fujino M, Funeshima N, Katsuma S, Shiojima S, Yamada M, Okuyama T, Suzuki S, Tsujimoto G (2003) Gene expression profile in the regenerating rat liver after partial hepatectomy. J Hepatol 38:784–792
Grasl-Kraupp B, Luebeck G, Wagner A, Low-Baselli A, de Gunst M, Waldhor T, Moolgavkar S, Schulte-Hermann R (2000) Quantitative analysis of tumor initiation in rat liver: role of cell replication and cell death (apoptosis). Carcinogenesis 21:1411–1421
Haag-Gronlund M, Conolly R, Scheu G, Warngard L, Fransson-Steen R (2000) Analysis of rat liver foci growth with a quantitative two-cell model after treatment with 2,4,5,3′,4′-pentachlorobiphenyl. Toxicol Sci 57:32–42
Imai T, Masui T, Ichinose M, Nakanishi H, Yanai T, Masegi T, Muramatsu M, Tatematsu M (1997) Reduction of glutathione S-transferase P-form mRNA expression in remodeling nodules in rat liver revealed by in situ hybridization. Carcinogenesis 18:545–551
Ito N, Tatematsu M, Hasegawa R, Tsuda H (1989) Medium-term bioassay system for detection of carcinogens and modifiers of hepatocarcinogenesis utilizing the GST-P positive liver cell focus as an endpoint marker. Toxicol Pathol 17:630–641
Kato M, Popp JA, Conolly RB, Cattley RC (1993a) Relationship between hepatocyte necrosis, proliferation, and initiation induced by diethylnitrosamine in the male F344 rat. Fundam Appl Toxicol 20:155–162
Kato T, Imaida K, Ogawa K, Hesegawa R, Shirai T, Tatematsu M (1993b) Three-dimensional analysis of glutathione S-transferase placental form-positive lesion development in early stages of rat hepatocarcinogenesis. Jpn J Cancer Res 84:1252–1257
Krutovskikh V (2002) Implication of direct host-tumor intercellular interactions in non-immune host resistance to neoplastic growth. Semin Cancer Biol 12:267–276
Liljegren G, Hardell L, Lindstrom G, Dahl P, Magnuson A (1998) Case-control study on breast cancer and adipose tissue concentrations of congener specific polychlorinated biphenyls, DDE and hexachlorobenzene. Eur J Cancer Prev 7:135–140
Luebeck EG, Buchmann A, Stinchcombe S, Moolgavkar SH, Schwarz M (2000) Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on initiation and promotion of GST-P-positive foci in rat liver: a quantitative analysis of experimental data using a stochastic model. Toxicol Appl Pharmacol 167:63–73
Luebeck EG, Grasl-Kraupp B, Timmermann-Trosiener I, Bursch W, Schulte-Hermann R, Moolgavkar SH (1995) Growth kinetics of enzyme-altered liver foci in rats treated with phenobarbital or alpha-hexachlorocyclohexane. Toxicol Appl Pharmacol 130:304–315
Moolgavkar SH, Dewanji A, Venzon DJ (1988) A stochastic two-stage model for cancer risk assessment. I. The hazard function and the probability of tumor. Risk Anal 8:383–392
Moolgavkar SH, Luebeck EG (1992) Interpretation of labeling indices in the presence of cell death. Carcinogenesis 13:1007–1010
Moolgavkar SH, Luebeck EG, Buchmann A, Bock KW (1996) Quantitative analysis of enzyme-altered liver foci in rats initiated with diethylnitrosamine and promoted with 2,3,7,8-tetrachlorodibenzo-p-dioxin or 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol 138:31–42
Moolgavkar SH, Luebeck EG, de Gunst M, Port RE, Schwarz M (1990) Quantitative analysis of enzyme-altered foci in rat hepatocarcinogenesis experiments-I. Single agent regimen. Carcinogenesis 11:1271–1278
NTP (2004) Toxicology and carcinogenesis studies of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) (CAS No. 57465–28-8) in female Harlan Sprague-Dawley rats (gavage studies). National Toxicology Program, Research Triangle Park, NC
Omori Y, Zaidan Dagli ML, Yamakage K, Yamasaki H (2001) Involvement of gap junctions in tumor suppression: analysis of genetically-manipulated mice. Mutat Res 477:191–196
Ou YC, Conolly RB, Thomas R, Gustafson DL, Long ME, Dovrev ID, Chubb LS, Xu Y, Lapidot S, Andersen ME, Yang RSH (2003) Stochastic simulation of hepatic preneoplasic foci development for four chlorobenzene congeners in a medium-term bioassay. Toxicol Sci 73:301–314
Ou YC, Conolly RB, Thomas RS, Xu Y, Andersen ME, Chubb LS, Pitot HC, Yang RS (2001) A clonal growth model: time-course simulations of liver foci growth following penta- or hexachlorobenzene treatment in a medium-term bioassay. Cancer Res 61:1879–1889
Plante I, Charbonneau M, Cyr DG (2002) Decreased gap junctional intercellular communication in hexachlorobenzene-induced gender-specific hepatic tumor formation in the rat. Carcinogenesis 23:1243–1249
Portier CJ, Sherman CD, Kohn M, Edler L, Kopp-Schneider A, Maronpot RM, Lucier G (1996) Modeling the number and size of hepatic focal lesions following exposure to 2,3,7,8-TCDD. Toxicol Appl Pharmacol 138:20–30
Saltykov SA (1967) The determination of the size distribution of particles in an opaque material from a measurement of the size distribution of their sections. In: Elias H (ed) Proceedings of the second international congress for stereology, Chicago, April 8–13. Springer, Berlin, pp 163–173
Satoh K, Hatayama I, Tateoka N, Tamai K, Shimizu T, Tatematsu M, Ito N, Sato K (1989) Transient induction of single GST-P positive hepatocytes by DEN. Carcinogenesis 10:2107–2111
Smith AG, Francis JE, Dinsdale D, Manson MM, Cabral JR (1985) Hepatocarcinogenicity of hexachlorobenzene in rats and the sex difference in hepatic iron status and development of porphyria. Carcinogenesis 6:631–636
Thomas RS (1998) The use of biologically-based models for integrating short-term cancer bioassays, mechanisms of action, and target tissue dosimetry: application to pentachlorobenzene. Ph.D. Dissertation, Department of Environmental Health, Colorado State University, Ft. Collins
Thomas RS, Conolly RB, Gustafson DL, Long ME, Benjamin SA, Yang RS (2000) A physiologically based pharmacodynamic analysis of hepatic foci within a medium-term liver bioassay using pentachlorobenzene as a promoter and diethylnitrosamine as an initiator. Toxicol Appl Pharmacol 166:128–137
Weibel ER, Staubli W, Gnagi HR, Hess FA (1969) Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol 42:68–91
Xu YH, Pitot HC (2003) An improved stereologic method for three-dimensional estimation of particle size distribution from observations in two dimensions and its application. Comput Methods Programs Biomed 72:1–20
Yang RSH (1994) Introduction to the toxicology of chemical mixtures. In: Yang RSH (ed) Toxicology of chemical mixtures: case studies, mechanisms, and novel approaches. Academic, San Diego, pp 1–10
Yang RSH (1997) Toxicologic interactions of chemical mixtures. In: Bond J (ed) Comprehensive toxicology, Vol. 1, General principles, toxicokinetics, and mechanisms of toxicity. Elsevier, Oxford, pp 189–203
Yusuf A, Rao PM, Rajalakshmi S, Sarma DS (1999) Development of resistance during the early stages of experimental liver carcinogenesis. Carcinogenesis 20:1641–1644
Acknowledgments
This study is supported by the NIOSH/CDC grant 1 RO1 OH07556, NIEHS Training Grant 1 T32 ES 07321, and scholarships from U.S. Fulbright Foundation and Naresuan University, Thailand. The authors thank Dr. Ying C. Ou for her help with the computer code and thank Ms. Traci Nichols, Drs. Todd Painter and Charles Dean, and other colleagues in the Quantitative and Computational Toxicology Group for their excellent technical assistance. The support on statistical analysis of the experimental data from Dr. Xiaohui Xu is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, Y., Lohitnavy, M., Reddy, M. et al. Quantitative analysis of liver GST-P foci promoted by a chemical mixture of hexachlorobenzene and PCB 126: implication of size-dependent cellular growth kinetics. Arch Toxicol 82, 103–116 (2008). https://doi.org/10.1007/s00204-007-0238-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-007-0238-x