Abstract
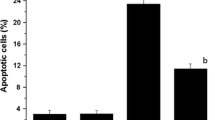
Despite the significant anti-tumor activities, cyclophosphamide (CP) also shows cytotoxicity to normal cells. In order to explore the protective effects of drugs against CP-induced adverse effects, 20(S)-ginsenoside Rg3 was tested for its possibly protective activities on CP-induced DNA damage and cell apoptosis in mouse bone marrow cells or peripheral lymphocyte cells. In the current study, the alkaline single cell gel electrophoresis (comet assay), flow cytometry assay with annexin V-FITC/PI and AO/EB staining assay were employed to measure DNA strand breakage and cell apoptosis, respectively. The activities of SOD and GPx and the contents of MDA were also tested by the various colormetric methods. The results showed that CP at a dose of 100 mg/kg, i.p. significantly caused DNA damages in both mouse bone marrow cells and peripheral lymphocyte cells, and markedly inhibited the activities of GPx and SOD and increased MDA contents in mouse blood. Moreover, CP at a dose of 200 mg/kg, i.p. triggered apoptosis in mouse bone marrow cells. On the other hand, 20(S)-ginsenoside Rg3 orally administered at a dose of 20 mg/kg to the animals once a day for 2 days significantly inhibited CP-induced DNA damages in mouse bone marrow cells and peripheral lymphocyte cells, decrease the apoptotic numbers of bone marrow cells, antagonized the reduction of the activities of SOD and GPx, and the increase in MDA contents. In conclusion, 20(S)-ginsenoside Rg3 showed the significant protective effects on CP-induced cell DNA damage and apoptosis. These effects might be partially attributed to its protective actions against CP-induced oxidative stress.



Similar content being viewed by others
References
Anderson HC, Chen H, Pellet LT, Tappel AL (1993) Ferousiron-induced oxidation in chicken liver slices as measured by hemichrome formation and thiobarbituric acid reactive substances: effect of dietary vitamin E and beta-carotene. Free Radic Biol Med 15:35–48
Bae EA, Han MJ, Shin YW, Kim DH (2006) Inhibitory effects of Korean red ginseng and its genuine constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive cutaneous anaphylaxis reaction and contact dermatitis models. Biol Pharm Bull 29:1862–1867
Brock N, Stekar J, Pohl J, Niemeyer U, Scheffter G (1979) Acrolein, the causative factor of urotoxic side-effects of cyclophosphamide, ifosfamide, trofosfamide and sufosfamide. Arzneimittelforschung 29:659–661
Bullock G, Tang C, Tourkina E, Ibrado AM, Lutzky J, Huang Y, Mahoney ME, Bhalla K (1993) Effect of combined treatment with interleukin-3 and interleukin-6 on 4- hydroperoxycyclo-phosphamide-induced programmed cell death or apoptosis in human myeloid leukemia cells. Exp Hematol 21:1640–1647
Cai L, Hales BF, Robaire B (1997) Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod 56:1490–1497
Colvin OM (1999) An overview of cyclophosphamide development and clinical applications. Curr Pharm Des 5:555–560
Crook TR, Souhami RL, McLean AEM (1986) Cytotoxicity, DNA cross-linking and single strand breaks induced by activated cyclophosphamide and acrolein in human leukemia cells. Cancer Res 46:5029–5034
Dumontet C, Drai J, Thieblemont C, Hequet O, Espinouse D, Bouafia F, Salles G, Coiffier B (2001) The superoxide dismutase content in erythrocytes predicts short-term toxicity of high-dose cyclophosphamide. Br J Haematol 112:405–409
Ferguson LR, Pearson AE (1996) The clinical use of mutagenic anticancer drugs. Mutat Res 355:1–12
Fraiser LH, Kanekal S, Kehrer JP (1991) Cyclophosphamide toxicity. Drugs 42:781–795
Fridovich I (1975) Superoxide dismutases. Annu Rev Biochem 44:147–159
Friedman OM, Wodinsky I, Mytes A (1976) Cyclophosphamide-related phosphoramide mustards-recent advances and historical perspective. Cancer Treat Rep 60:337–346
Halliwell B, Gutteridge JM (1989) Free radicals in biology and medicine, 2nd edn. Clarendon Press, Oxford
Horn PL, Thompson WD (1988) Exposure to chemotherapeutic agents and the risk of a second breast cancer: preliminary findings. Yale J Biol Med 61:223–231
Kang XM, Zhang QY, Tong DD, Zhao W (2005) Experimental study on anti-angiogenesis in mice with Lewis lung carcinoma by low-dose of cyclophosphamide combined with ginsenoside Rg3. Zhongguo Zhong Xi Yi Jie He Za Zhi 25:730–703
Kim JT, Park SH, Kim SK, Kwon EY, Do MH, Hwang TH (2002) Potential role of homer-2a on cutaneous vascular anomaly. J Korean Med Sci 17:636–640
Lecoeur H, Prevost MC, Gougeon ML (2001) Oncosis is associated with exposure of phosphatidylserine residues on the outside layer of the plasma membrane: a reconsideration of the specificity of the annexin V/propidium iodide assay. Cytometry 44:65–72
Lishi H, Tatsuta M, Baba M, Uehara H, Nakaizumi A, Shinkai K, Akedo H, Funai H, Ishiguro S, Kitagawa I (1997) Inhibition by ginsenoside Rg3 of bombesin-enhanced peritoneal metastasis of intestinal adenocarciomas induced by azoxymethane in Wister rats. Clin Exp Metastasis 15:603–611
Liu WK, Xu SX, Che CT (2000) Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci 67:1297–1306
Nishikimi M (1975) Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun 63:463–468
Pan ZM, Ye DF, Xie X, Chen HZ, Lu WG (2002) Antiangiogenesis of ginsenoside Rg3 in severe combined immunodeficient mice with human ovarian carcinoma. Chin J Obstet Gynecol 37:227–230
Patel JM, Block ER (1985) Cyclophosphamide-induced depression of the antioxidant defense mechanisms of the lung. Exp Lung Res 8:153–165
Ponticelli C, Passerini P (1991) Alkylating agents and purine analogues in primary glomerulonephritis with nephrotic syndrome. Nephrol Dial Transplant 6:381–388
Rekha PS, Kuttan G, Kuttan R (2001) Effect of Brahma Rasayana on antioxidant systems and cytokine levels in mice during cyclophosphamide administration. J Exp Clin Cancer Res 20:219–223
Rotruk JT, Popew AC, Ganther HE, Swason AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Shinkai K, Akedo H, Mukai M, Imamura F, Isoai A, Kobayashi M, Kitagawa I (1996) Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Jpn J Cancer Res 87:357–362
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Biol 175:184–191
Sladek NE (1971) Metabolism of cydophosphamide by rat hepatic microsomes. Cancer Res 31:901–908
Smith JR, Kay NK, Gottlieb AJ, Oski FA (1975) Abnormal erythrocyte metabolism in hepatic disease. Blood 46:955–946
Tian J, Fu F, Geng M, Jiang Y, Yang J, Jiang W, Wang C, Liu K (2005) Neuroprotective effect of 20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci Lett 374:92–97
Zhang Q, Kang X, Zhao W (2006) Antiangiogenic effect of low-dose cyclophosphamide combined with ginsenoside Rg3 on Lewis lung carcinoma. Biochem Biophys Res Commun 342:824–828
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Q.H., Wu, C.F., Duan, L. et al. Protective effects of ginsenoside Rg3 against cyclophosphamide-induced DNA damage and cell apoptosis in mice. Arch Toxicol 82, 117–123 (2008). https://doi.org/10.1007/s00204-007-0224-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-007-0224-3