Abstract
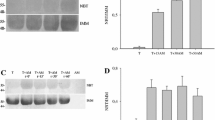
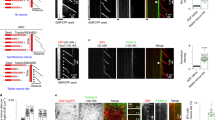
This study investigated the hypothesis that the chromosomal genotoxicity of inorganic mercury results from interaction(s) with cytoskeletal proteins. Effects of Hg2+ salts on functional activities of tubulin and kinesin were investigated by determining tubulin assembly and kinesin-driven motility in cell-free systems. Hg2+ inhibits microtubule assembly at concentrations above 1 µM, and inhibition is complete at about 10 µM. In this range, the tubulin assembly is fully (up to 6 µM) or partially (~6–10 µM) reversible. The inhibition of tubulin assembly by mercury is independent of the anion, chloride or nitrate. The no-observed-effect-concentration for inhibition of microtubule assembly in vitro was 1 µM Hg2+, the IC50 5.8 μM. Mercury(II) salts at the IC50 concentrations partly inhibiting tubulin assembly did not cause the formation of aberrant microtubule structures. Effects of mercury salts on the functionality of the microtubule motility apparatus were studied with the motor protein kinesin. By using a “gliding assay” mimicking intracellular movement and transport processes in vitro, HgCl2 affected the gliding velocity of paclitaxel-stabilised microtubules in a clear dose-dependent manner. An apparent effect is detected at a concentration of 0.1 µM and a complete inhibition is reached at 1 μM. Cytotoxicity of mercury chloride was studied in V79 cells using neutral red uptake, showing an influence above 17 µM HgCl2. Between 15 and 20 µM HgCl2 there was a steep increase in cell toxicity. Both mercury chloride and mercury nitrate induced micronuclei concentration-dependently, starting at concentrations above 0.01 µM. CREST analyses on micronuclei formation in V79 cells demonstrated both clastogenic (CREST-negative) and aneugenic effects of Hg2+, with some preponderance of aneugenicity. A morphological effect of high Hg2+ concentrations (100 µM HgCl2) on the microtubule cytoskeleton was verified in V79 cells by immuno-fluorescence staining. The overall data are consistent with the concept that the chromosomal genotoxicity could be due to interaction of Hg2+ with the motor protein kinesin mediating cellular transport processes. Interactions of Hg2+ with the tubulin shown by in vitro investigations could also partly influence intracellular microtubule functions leading, together with the effects on the kinesin, to an impaired chromosome distribution as shown by the micronucleus test.







Similar content being viewed by others
References
Akiyama M, Oshima H, Nakamura M (2001) Genotoxicity of mercury used in chromosome aberration tests. Toxicol In Vitro 15:463–467
Al-Sabti K (1994) Micronuclei induced by selenium, mercury, methylmercury and their mixtures in binucleated blocked fish erythrocyte cells. Mutat Res 320:157–163
Boffetta P, Merler E, Vainio H (1993) Carcinogenicity of mercury and mercury compounds. Scand J Work Environ Health 19:1–7
Boffetta P, Garcia-Gomez M, Pompe-Kirn V, Zaridze D, Bellander T, Bulbulyan M, Caballero JD, Ceccarelli F, Colin D, Dizdarevic T, Espanol S, Kobal A, Petrova N, Sallsten G, Merler E (1998) Cancer occurrence among European mercury miners. Cancer Causes Control 9:591–599
Bolt HM (2003) Genotoxicity—threshold or not? Introduction of cases of industrial chemicals. Toxicol Lett 140/141:43–51
Bolt HM, Foth H, Hengstler JG, Degen GH (2004) Carcinogenicity categorisation of chemicals—new aspects to be considered. Toxicol Lett 151:29–42
Bonacker D, Stoiber T, Böhm KJ, Unger E, Degen GH, Thier R, Bolt HM (2004) Chromosomal genotoxicity of nitrobenzene and benzonitrile. Arch Toxicol 78:49–57
Borenfreund E, Babich H, Martin-Alguacil N (1988) Comparisons of two in vitro cytotoxicity assays—the neutral red (NR) and tetrazolium MTT test. Toxicol in Vitro 2:1–6
Böhm KJ, Stracke R, Unger E (2000) Speeding up kinesin-driven microtubule gliding in vitro by variation of cofactor composition and physicochemical parameters. Cell Biology International 24:335–341
Countryman PI, Heddle JA (1976) The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutation Res 41:321–332
Cross HJ, Smillie MV, Chipman JK, Fletcher AC, Levy LS, Spurgeon A, Fairhurst S, Howe A, Mason H, Northage C, Wright A (1995) Mercury and its inorganic divalent compounds. Criteria document for an occupational exposure limit. HSE Books, Health and Safety Executive, London, UK. ISBN 0-7176-10144
Decordier I, Dillen L, Cundari E, Kirsch-Volders M (2002) Elimination of micronucleated cells by apoptosis after treatment with inhibitors of microtubules. Mutagenesis 17:337–344
De Flora S, Bennicelli C, Bagnasco M (1994) Genotoxicity of mercury compounds. A review. Mutat Res 317:57–79
DFG (1999) Quecksilber und anorganische Quecksilberverbindungen. In: Greim H (ed) Gesundheitsschädliche Arbeitsstoffe. Toxikologisch-arbeitsmedizinische Begründungen von MAK-Werten. 28. WILEY-VCH, Weinheim, Lieferung, pp 1–42
Dieter MP, Boorman GA, Jameson CW, Eustis SL, Uraith LC (1992) Development of renal toxicity in F344 rats gavaged with mercuric chloride for 2 weeks, or 2, 4, 6, 15, and 24 months. J Toxicol Environ Health 36:319–340
Duhr EF, Pendergrass JC, Slevin JT, Haley BE (1993) HgEDTA complex inhibits GTP interactions with the E-site of brain beta-tubulin. Toxicol Appl Pharmacol 122:273–280
Elhajouji A, Van Hummelen P, Kirsch-Volders M (1995) Indications for a threshold of chemically-induced aneuploidy in vitro in human lymphocytes. Environ Mol Mutagen 26:292–304
Fenech M (1993) The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res 285:35–44
Gaskin F, Cantor CR, Shelanski ML (1974) Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol 89:737–755
Hengstler JG, Bogdanffy MS, Bolt HM, Oesch F (2003) Challenging dogma: thresholds for genotoxic carcinogens? The case of vinyl acetate. Annu Rev Pharmacol Toxicol 43:485–520
Keates RAB, Yott B (1984) Inhibition of microtubule polymerization by micromolar concentrations of mercury(II). Can J Biochem Cell Biol 62 814–818
Kirsch-Volders M, Aardema M, Elhajouji A (2000) Concept of threshold in mutagenesis and carcinogenesis. Mutation Res 464:3–11
Kirsch-Volders M, Vanhauwaert A, Eichenlaub-Ritter U, Decordier I (2003) Indirect mechanisms of genotoxicity. Toxicol Lett 140/141:63–74
Kuznetsov SA, Gelfand VI (1986) Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci U S A 83:8530–8534
Léonard A (1988) Mechanisms in metal genotoxicity: the significance of in vitro approaches. Mutation Res 198:321–326
Liliom K, Wagner G, Pacz A, Cascante M, Kovacs J, Ovadi J (2000) Organization-dependent effects of toxic bivalent ions microtubule assembly and glycolysis. Eur J Biochem 267:4731–4739
Matsuoka A, Yamazaki N, Suzuki T, Hayashi M, Sofuni T (1992) Evaluation of the micronucleus test using a Chinese hamster cell line as an alternative to the conventional in vitro chromosomal aberration test. Mutation Res 272:223–236
Miller BM, Adler ID (1990) Application of antikinetochore antibody staining (CREST staining) to micronuclei in erythrocytes induced in vivo. Mutagenesis 5:411–415
Miura K, Inokawa M, Imura N (1984) Effects of methylmercury and some metal ions on microtubule networks in mouse glioma cells and in vitro tubulin polymerization. Toxicol Appl Pharmacol 73:218–231
National Toxicology Program (1993) Toxicology and carcinogenesis studies of mercuric chloride (CAS no. 7487-94-7) in F344 rats and B6C3F1 mice (gavage studies) Natl Toxicol Program Tech Rep Ser 408:1–260
Pratt IS, Barron T (2003) Regulatory recognition of indirect genotoxicity mechanisms in the European Union. Toxicol Lett 140/141:53–62
Rao MV, Chinoy NJ, Suthar MB, Rajvanshi MI (2001) Role of ascorbic acid on mercuric chloride-induced genotoxicity in human blood cultures. Toxicol In Vitro 15:649–654
Renzi L, Pacchierotti F, Russo A (1996) The centromere as a target for the induction of chromosome damage in resting and proliferating mammalian cells: assessment of mitomycin C-induced genetic damage at kinetochores and centro-meres by a micronucleus test in mouse splenocytes. Mutagenesis 11:133–138
Russo A, Stocco A, Majone F (1992) Identification of kinetochore-containing (CREST+) micronuclei in mouse bone marrow erythrocytes. Mutagenesis 7:195–197
Schoeny R (1996) Use of genetic toxicology data in U.S. EPA risk assessment: the mercury study report as an example. Environ Health Perspect 104[Suppl 3]: 663–673
Schuler M, Rupa DS, Eastmond DA (1997) A critical evaluation of centromeric labeling to distinguish micronuclei induced by chromosomal loss and breakage in vitro. Mutation Res 392:81–95
Schurz F, Sabater-Vilar M, Fink-Gremmels J (2000) Mutagenicity of mercuric chloride and mechanism of cellular defence: the role of metal-binding proteins. Mutagenesis 15:525–530
Shelanski ML, Gaskin F, Cantor CR (1973) Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A 70:765–768
Stoiber T, Degen GH, Bolt HM, Unger E (2004) Interaction of mercury(II) with the microtubule cytoskeleton in IMR-32 neuroblastoma cells. Toxicol Lett 151:99–104
Thier R, Bonacker D, Stoiber T, Böhm KJ, Wang M, Unger E, Bolt HM, Degen G (2003) Interaction of metal salts with cytoskeletal motor protein systems. Toxicol Lett 140/141:75–81
Unger E, Böhm KJ, Müller H, Grossmann H, Fenske H, Vater W (1988) Formation of double-walled microtubules and multilayered tubulin sheets by basic proteins. Eur J Cell Biol 46:98–104
Vogel DG, Margolis RL, Mottet NL (1989) Analysis of methyl mercury binding sites on tubulin subunits and microtubules. Pharmacol Toxicol 64:196–201
Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A 72:1858–1862
Weiss DG, Maile W (1992) Principles, practice and applications of video-enhanced contrast microscopy. In: Shotton DM (ed) Electronic light microscopy. Wiley-Liss, New York, pp 105–140
Acknowledgements
The studies were supported by CEFIC (CEFIC/LRI: CC-1FOAR-0003). The dedicated stewardship by Dr. A. Sarrif is very much appreciated. The authors thank H. Wolfram for performing part of the in vitro assays. The Ph.D. thesis of D.B., submitted to the Faculty of Science of the Heinrich-Heine University, Düsseldorf, Germany, contains part of the data of the present publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonacker, D., Stoiber, T., Wang, M. et al. Genotoxicity of inorganic mercury salts based on disturbed microtubule function. Arch Toxicol 78, 575–583 (2004). https://doi.org/10.1007/s00204-004-0578-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-004-0578-8