Abstract.
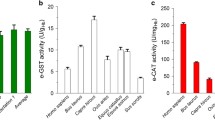
The human glutathione S-transferase hGSTT1-1 is characterized by a polymorphism displaying three phenotypes: the "non-conjugator" (NC) phenotype expresses no or only a residual activity due to a homozygous deletion of the hGSTT1 gene, a medium hGSTT1-1 activity can be demonstrated for the "low conjugator" (LC) phenotype as the heterozygous bearer of one hGSTT1 allele, and a high hGSTT1-1 activity is detected for the "high conjugator" (HC) phenotype as the homozygous bearer of two hGSTT1 alleles. We have developed a routine ex vivo photometric phenotyping procedure based on the determination of bromide release rates from the hGSTT1-1-catalyzed glutathione conjugation of the substrate methyl bromide in EDTA blood samples under standard conditions (1,000 ppm methyl bromide, 10 min incubation). The bromide release rates were standardized to the hemoglobin (Hb) value. Twenty-six individuals were phenotyped following the new procedure. Four individuals were classified as NCs (24–33 pmol Br–/mg Hb per min), 21 individuals were regarded as LCs (107–206 pmol Br–/mg Hb per min) and one person of the study group was designated HC (236 pmol Br–/mg Hb per min). The results were validated by qualitative hGSTT1 genotyping and demonstrated a 100% match for conjugators and non-conjugators. A second HPLC phenotyping routine procedure based on the formation of S-methylglutathione from methyl chloride in erythrocyte lysate incubations (Müller et al. 2001, Arch Toxicol 74:760–767) was established and validated by genotyping. The phenotyping results obtained with both methods were correlated, resulting in a good correlation with R 2=0.64 (y=0.8997x+51.535). Three distinct phenotype clusters for NCs, LCs and HCs, consistent with the proposed genetics, were demonstrated. Assay-dependent storage experiments revealed an excellent stability of the hGSTT1-1 activity. In conclusion, the evaluated methods provide powerful tools for determination of hGSTT1-1 activity as a clinical parameter.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Müller, M., Bünger, J., Voss, M. et al. Phenotyping of human glutathione S-transferase hGSTT1-1: a comparison of two ex vivo routine procedures. Arch Toxicol 76, 634–642 (2002). https://doi.org/10.1007/s00204-002-0391-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00204-002-0391-1