Abstract.
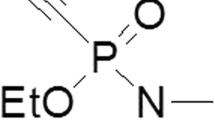
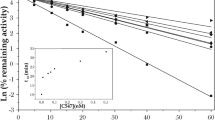
Standard treatment of poisoning by organophosphates (OP) includes the administration of an antimuscarinic agent, e.g. atropine, and of an acetylcholinesterase (AChE) reactivator (oxime). The presently available oximes, obidoxime and pralidoxime (2-PAM), are considered to be insufficient for highly toxic OPs, e.g. sarin. In the past decades numerous oximes were prepared and tested for their efficacy in OP poisoning, mostly in animal experiments. However, data indicate that the reactivating potency of oximes may be different in humans and animal species, which may hamper the extrapolation of animal data to humans and may pose a problem in the drug licensing of new compounds. In order to provide data for a better evaluation of the reactivating potency of oximes, experiments were undertaken to determine the reactivation rate constants of several oximes with human, rabbit, rat and guinea-pig AChE inhibited by the OPs sarin, cyclosarin and VX. The results show marked differences among the species, depending on the inhibitor and on the oxime, and indicate that the findings from animal experiments need careful evaluation before extrapolating these data to humans.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Worek, .F., Reiter, .G., Eyer, .P. et al. Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch Toxicol 76, 523–529 (2002). https://doi.org/10.1007/s00204-002-0375-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00204-002-0375-1