Abstract
Shiga toxin-producing E. coli (STEC) are an important cause of foodborne illness in humans with infections ranging from mild non-bloody diarrhea to bloody diarrhea (BD) and hemolytic uremic syndrome (HUS). This study aimed to investigate the distribution of STEC in shellfish from coastal shores of Lake Timsah in Ismailia Governorate, Egypt and its probable hazard to seafood consumers. Samples from the external surface and tissues of shrimp (n = 45), crabs (n = 45), and oysters (n = 45) batches were examined bacteriologically for the presence of STEC and tested for their antibiotic sensitivity. Moreover, occurrence of virulence genes was determined via detection of stx1, stx2 and eaeA genes using PCR. Overall, E. coli and presumptive STEC isolates (from CHROMagar) were identified from the surface (55.6 and 5.9%) and tissues (42.2 and 8.9%) of the examined shellfish batches, respectively. Five STEC isolates had been confirmed and found belonging to O26:H11, O125:H6, O146:H21, and O159 serogroups, those were 4 isolates from tissues of the three shellfish species and one isolate from the crab surface. The STEC isolates were multi-drug resistant, showing complete resistance to; penicillins, amoxycillin/clavulanic acid, colistin, fosfomycin, ceftriaxone, ciprofloxacin, and tetracycline, however, they were sensitive to gentamycin except O159 serogroup. The current study revealed low level of contamination of shellfish from coastal shores of Lake Timsah with STEC, however, it also highlights the extreme level of antimicrobial resistance exhibited by the presumptive and confirmed STEC isolates which is very hazardous for seafood consumers in the study area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Escherichia coli has been long thought to be a sign of faecal contamination in water and food. There are over 700 E. coli serotypes, however, the majority of which are non-pathogenic and present as commensals in the human and animal gastrointestinal tract. A limited number of E. coli pathotypes have developed the potential to produce a wide range of illnesses in humans ranged from wound infections to deadly meningitis (Jang et al. 2017). Intestinal E. coli can be classified into at least five types, with the Shiga toxin-producing E. coli (STEC), (known also as enterohemorrhagic E. coli, EHEC), being the most dangerous (Prakasan et al. 2018).
Shellfish including bivalves and gastropod mollusks (cockles, oysters, mussels, clams, periwinkles, and sea-snails), as well as, crustacean shellfish (lobster, crab, and shrimp) are preferable as rich sources of protein for humans, however, their contamination with bacteria found in human feces is still considered a health risk (Costa 2013). STEC can infect individuals inhabiting marine environment through contaminated seafood and by contaminated water and food; where bacteria colonize the intestinal microvilli before attacking the central nervous system and kidney cells (Kamala et al. 2022; Sujatha et al. 2011).
STEC infections cause 100,000 illnesses, 3000 hospitalizations, and 90 fatalities per year in the United States alone (Gomes et al. 2016). The development of one or more Shiga toxins is the STEC’s key virulence factors. There are two types of Shiga toxins (Stx); Stx1, which is nearly identical to the toxins generated by Shigella dysenteriae 1, and Stx2, that is roughly 60% similar to Stx1 (Steiner 2016). Although the formation of one or more Shiga toxins is required to produce illness, the production of Stx2 is more closely linked to the severity of diseases like haemolytic uremic syndrome (HUS) and hemorrhagic colitis (HC) (Melton-Celsa 2014).
Additional to the synthesis of one more Shiga toxin, numerous supplementary virulence factors are known to influence STEC pathogenicity in human infections. The ability to create attachment and effacement lesions (A/E lesions) is one of the significant virulence features of STEC (Smith et al. 2014). Although the exact role of these virulence factors is unknown (Kamala et al. 2022), these virulence factors have been found in STEC reported from clinical cases of HC and HUS on a regular basis, if not always. Despite O157:H7 is the most prevalent serotype that has been linked to the majority of large-scale STEC outbreak infections, non-O157 serotypes such O26, O91, O45, O111, O103, O145, and O121 are also involved in serious human infections (Luna-Gierke et al. 2014).
Lake Timsah is one of the important coastal areas in Ismailia Governorate, Egypt, with a salinity ranges from 20–40% in surface areas and more than 40% in deeper ones (El-Serehy et al. 2018). Although ruminants are the main reservoirs for STEC, other sources including wildlife, marine environment, and aquaculture could be possible spillover hosts for these bacteria (Kamala et al. 2022; Kim et al. 2020). Although previous studies have documented presence of several pathogens, there have been few reports on STEC spreading densities from coastal and marine environment (Baliere et al. 2015; Kamala et al. 2022; Martin et al. 2019; Prakasan et al. 2018). The present study aims to investigate to which level STEC and their virulence factors are present on the surfaces and accumulated in the tissues of shellfish at harvesting areas of Lake Timsah, Ismailia, Egypt.
Material and methods
Ethical statement
Sample collection and laboratory procedures for the present study was reviewed and approved by the Ethical Committee of Faculty of Veterinary Medicine, Suez Canal University, Egypt (No. 2017003).
Sample collection and preparation
A total of 135 pooled fresh marine shellfish batches (45 shrimp, 45 crabs, and 45 oysters) were collected from different locations along the Shore of Lake Timsah at Ismailia governorate. Collection locations were the common harvesting sites where local fishermen collect the shellfish to be marketed for consumers. Samples from each collection batch were kept separately in a plastic bag and transferred in an ice box within 1 hour of collection to the Zoonoses Department’s Laboratory, Faculty of Veterinary Medicine, Suez Canal University for processing and isolation of E. coli.
Isolation and identification of E. coli and STEC detection
Soft tissue samples; about 25 g of each shellfish species batch comprised of 7–15 individual shrimp, crab or oyster, were pooled to represent one sample, placed in a sterile stomacher bag with 225 ml E. coli broth (Biolife, Italiana) and homogenized in stomacher (Lab. Blender 400, Seward Lab, London) for 3 min. 1 mL of the homogenized tissue was incubated with 9 mL of E. coli broth at 37 ℃ for 24 h (Doyle et al. 2020). Moreover, sterile swabs were rolled over the outer surface of the pooled shellfish samples, immersed in E. coli broth (Biolife, Italiana) and incubated at 37 ℃ for 24 h. Loopfuls from each surface swab and tissue’s E. coli broth culture were sub-cultured on MacConkey agar (Himedia, India) and EMB agar (Himedia, India) that were incubated at 37 ℃ for 24 h. Typical E. coli colonies on EMB (greenish metallic sheen colonies in reflected light) were re-cultured until pure colonies were obtained (Doyle et al. 2020). Isolates were biochemically identified using IMVIC tests and isolation on TSI as previously described (Thampuran et al. 2005). For selective STEC detection, pure identified E. coli colonies were grown on CHROMagar STEC agar medium (CHROMagar Microbiology, Paris, France) and incubated at 37 ℃ for 24 h (Meng et al. 2012).
Serological identification of presumptive STEC isolates
Presumptive STEC isolates successfully grown on CHROMagar agar medium (mauve colonies) were serologically identified in the Food analysis Center at Faculty of Veterinary Medicine, Benha University. Rapid diagnostic E. coli polyvalent antisera sets against O and H antigen for identification of the Enteropathogenic types were used as previously described (Kok et al. 1996).
Virulence profile of presumptive STEC isolates from shellfish
Virulence of presumptive STEC isolates were assessed using PCR for 3 virulence genes stx1, stx2, and eaeA (Table 1). The QIAamp DNA Mini kit (Qiagen, Germany, GmbH) was used to extract bacterial DNA based on the manufacturer’s instructions. Duplex PCR reactions for stx1 and stx2 was performed in a 50 µl reaction mixture, containing 25 µl of EmeraldAmp Mix PCR Master Mix (Takara, Japan), 1 µl of each primer (20 pmol concentration) (Metabion, Germany), 15 µl of molecular grade water, and 6 µl of bacterial DNA. Moreover, a uniplex PCR reactions for eaeA attachment gene was performed in a 25 µl reaction mixture, containing 12.5 µl of EmeraldAmp Mix PCR Master Mix (Takara, Japan), 1 µl of each primer (20 pmol concentration) (Metabion, Germany), 5.5 µl of molecular grade water, and 5 µl of bacterial DNA. The PCR reactions were performed in an Applied biosystem 2720 thermal cycler. PCR products (20 µl) were separated by electrophoresis at room temperature against 100 bp ladder (Fermentas, Germany) through 1.5% agarose gel (Applichem, Germany, GmbH) in 1 × TBE buffer. The gel was then photographed using Gel Documentation System (Alpha Innotech, Biometra).
Antimicrobial sensitivity of presumptive STEC isolates from shellfish
Identified STEC isolates were tested for their sensitivity against 12 antibiotics from different antimicrobial classes using disc diffusion method on Muller Hinton agar (Oxoid, UK). Antibiotic discs (Oxoid, UK) with following concentrations were used: ampicillin (AM) 10 µg, piperacillin (PRL) 100 µg, amoxycillin/clavulanic acid (AMC) 30 µg, ceftriaxone (CTR) 30 µg, azithromycin (AT) 15 µg, chloramphenicol (C) 30 µg, ciprofloxacin (CIP) 5 µg, colistin (CT) 10 µg, fosfomycin (FF) 50 µg, gentamycin (Gen) 10 µg, trimethoprim sulphamethoxazole (SXT) 25 µg, and tetracycline (TE) 30 µg. Pure freshly grown STEC isolates were diluted in 5 ml Muller Hinton broth (Oxoid, UK) to a density = 0.5 MacFarlane standard. Sterile swabs were used to evenly streak the surface of MHA plates and left for 30 min. to dry. Using sterile forceps, antibiotic discs were placed firmly to the plate and incubated at 37℃ for 18 h before measuring the inhibition zone as recommended by the manufacturer and Clinical and Laboratory Standard Institute (CLSI 2020), where isolate were categorized as resistant, intermediate or sensitive.
Results
Out of the examined shellfish batches from (45 oyster, 45 crabs, and 45 shrimp), the surface had higher E. coli and presumptive STEC detection rate (55.6 and 5.93%) than the edible tissues (42.2 and 8.89%). Among the total E. coli isolates, 20 presumptive STEC isolates showed characteristic mauve colonies on CHROMagar STEC medium; 12 isolates from tissues (8.89%) and 8 isolates from the external surface (5.93%) of the examined shellfish specimens. The recovery rate of E. coli and presumptive STEC from surface and tissues among different shellfish species (shrimp, crabs, and oysters) was nearly similar, except the tissues from oysters had the highest occurrence (11.11%) (Table 2).
Recovered presumptive STEC isolates belonged to 9 serotypes (O26:H11, O55:H7, O103:H2, O44:H18, O117:H4, O125:H6, O128:H2, O146:H21, and O159) with O117:H4, O128:H2, O44:H18 were the most frequent serotypes (Table 2). Molecularly, 11 presumptive STEC isolates carried eaeA gene (55%), 3 isolates carried stx2 gene (15%) and only 2 isolates had stx1 (10%) virulence gene. Of these, three isolates carried eaeA mutually with either stx1 or stx2 (Table 2). Confirmed STEC isolates (5 isolates, 4 from tissues of oyster, crabs, and shrimp, and one from crab surface that were positive for stx1 or stx2) belonged to O26:H11, O125:H6, O146:H21, and O159 serogroups.
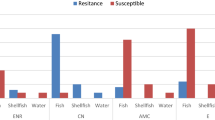
The overall antimicrobial sensitivity test results for presumptive STEC isolates is shown in (Table 3 and Fig. 1). The confirmed STEC isolates were multi-drug resistant (MDR), where isolates showed complete resistance to; ampicillin, piperacillin, amoxycillin/clavulanic acid, colistin, fosfomycin, ceftriaxone, ciprofloxacin, and tetracycline, however, they were sensitive to gentamycin except O159 serogroup.
ND not determined.
Discussion
Shellfish can concentrate many important pathogenic bacteria of human food borne transmission from the surrounding waterways by their filter-feeding activities (Walker et al. 2020), consequently, their consumption may result in food poisoning outbreaks.
Results from the present study revealed that, shellfish samples from coastal shores of Lake Timsah harbored virulent multi-drug resistant STEC (Table 2 & 3). The present findings showed higher E. coli detection rate from crabs’ tissues and surface samples (46.7 and 60%, respectively) than oysters and shrimp (40 and 53.3%, in both of them). The prevalence of E. coli from the surface was higher than that from the tissues in the 3 studied shellfish species. This may be because the surface is more exposed to contaminated surrounding water. A total of 150 E. coli isolates were recovered from 4 fresh shellfish species in Mumbai, India (Singh et al. 2020). Moreover, in another study, all the examined shellfish samples from landing centers and retail fish markets were positive for E. coli (100%) (Prakasan et al. 2018). In this regard, Martin and his co-authors reported that any edible marine shellfish species found exposed to fecal contamination, could be regarded as a potential source of STEC (Martin et al. 2019).
The significant increase in STEC outbreaks over the past decade as a consequence to ingestion of contaminated food or contact with animals and animal products is a public health issue (Kim et al. 2020). Out of the total E. coli isolates in this study, 5 STEC isolates were confirmed (4 from tissues of the three shellfish species and one from crab surface). Similarly, STEC (5%) were detected in shellfish samples from coastal harvesting areas in France (Baliere et al. 2015) and a very low level of STEC (3/269, 1.1%) was reported from Norwegian bivalves (Martin et al. 2019). However, a huge number, 3742 STEC isolates, were recently reported from fish, coastal waters, and sediments of Southeast Coast of India (Kamala et al. 2022). In Europe, besides O157:H7, 4 serotypes; O26:H11, O103:H2, O145:H28, and O111:H8 are the most commonly implicated in human STEC outbreaks, comprising the five highly pathogenic serotypes (EFSA 2013). In this regard, the first 2 of these serotypes had been detected from shrimp tissues and external surface in the present study, however, the O103:H2 didn’t carry any of the shiga toxin genes and only carried the eaeA gene (Table 2). Generally, E. coli organisms in fish and fishery products are very alarming for human health, as well as, for fish export earnings. Overall, the quality of fresh fish could be considered as a strong sanitary indicator for bacterial contamination (especially E. coli) and unhygienic conditions of water or fish aquarium (Jang et al. 2017), thus understanding food sources for STEC and possible reservoirs is very crucial.
STEC are identified as those strains that produce shiga toxins (encoded by stx1 and stx2 genes). Presence of virulence-associated genes is the primary differentiating feature between pathogenic and non-pathogenic E. coli strains. Using PCR, in the present study, eaeA was the most prevalent (55%) virulence gene in presumptive STEC isolates, however, stx1 and stx2 were detected in only 5 isolates; with 3 isolates (O159 and O146:H21) mutually carried eaeA with either stx1 or stx2. A higher level of eae (74.8%) and stx (30.3%) genes were detected in shellfish samples from coastal harvesting areas in France (Baliere et al. 2015). However, STEC from marine sediments and shellfish in Morocco lack the intimin eae gene (Bennani et al. 2011). In India, 1518 out of 3742 STEC isolates from fish, coastal waters, and sediments of Southeast Coast carried the stx1, stx2, and eae virulence genes (Kamala et al. 2022). Also, stx1, stx2, and eae virulence genes were detected at lower rate (19/269, 7%) from STEC isolates recovered from marine Norwegian bivalves (Martin et al. 2019). Presence of such virulent STEC isolates is of especial interest for shellfish consumers in the study area, as most of these edible shellfish species usually cooked by steaming and didn’t receive sufficient heat treatment during cooking.
Distribution of MDR bacteria is a global public health threat. Commercial fish could be a vehicle of antibiotic-resistant human pathogens that may be indirectly transmitted to human causing sever public health hazard (Singh et al. 2020). According to sensitivity testing results in the present study, all presumptive STEC isolates were multi-drug resistant (MDR) strains (they exhibited resistance to antibiotics from more than three antimicrobial classes). Similarly, all E. coli isolates from seafoods in Lagos Nigeria showed high level of resistance (85.7–96.1%) to tetracycline and trimethoprim, however, isolates were highly susceptible to ciprofloxacin, amikacin, imipenem and cefepime (Odumosu et al. 2021). In Mumbai, India, 71.58% of 475 E. coli isolates obtained from fresh seafood in retail markets exhibited high extended spectrum B-lactamase resistance; cefotaxime (95%), ceftazidime (90.29%), and cefpodoxime (90.88%), however, isolates showed considerable susceptibility to cefoxitin (66.76%), imipenem (74.41%), and meropenem (51.18%) (Singh et al. 2020). These findings are especially alarming due to the risk of disseminating multidrug-resistant E. coli strains to seafood consumer, thus improving the hygiene of coastal waters is quite essential.
Findings in the present study may suggest that shellfish could be possible spillover reservoirs of resistant and virulent STEC, and improperly heat-treated edible shellfish may be a probable source of infection for consumers in the study area. Additional future STEC surveillance and interdisciplinary coordinated efforts are essentially needed to protect coastal water and prevent STEC outbreaks associated with the human consumption of these invertebrates.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Baliere C, Rince A, Blanco J, Dahbi G, Harel J, Vogeleer P et al (2015) Prevalence and characterization of shiga toxin-producing and enteropathogenic escherichia coli in shellfish-harvesting areas and their watersheds. Front Microbiol 6:1356
Bennani M, Badri S, Baibai T, Oubrim N, Hassar M, Cohen N et al (2011) First detection of Shiga toxin-producing Escherichia coli in shellfish and coastal environments of Morocco. Appl Biochem Biotechnol 165(1):290–299
Bisi-Johnson MA, Obi CL, Vasaikar SD, Baba KA, Hattori T (2011) Molecular basis of virulence in clinical isolates of Escherichia coli and Salmonella species from a tertiary hospital in the Eastern Cape. South Africa Gut Pathog 3(1):9
CLSI. (2020) performance standards for antimicrobial susceptibility testing. 30th eds. CLSI supplement M100
Costa RA (2013) Escherichia coli in seafood: A brief overview. Adv Biosci Biotechnol 4:450–454
Dipineto L, Santaniello A, Fontanella M, Lagos K, Fioretti A, Menna LF (2006) Presence of Shiga toxin-producing Escherichia coli O157:H7 in living layer hens. Lett Appl Microbiol 43(3):293–295
Doyle MP, Diez-Gonzalez F, Hill C (2020) Food microbiology: fundamentals and frontiers: John Wiley & Sons. ASM Press
EFSA (2013) EFSA Panel on Biological Hazards (BIOHAZ); Scientific Opinion on VTEC-seropathotype and scientific criteria regarding pathogenicity assessment. EFSA J 11(4):3138
El-Serehy HA, Abdallah HS, Al-Misned FA, Al-Farraj SA, Al-Rasheid KA (2018) Assessing water quality and classifying trophic status for scientifically based managing the water resources of the Lake Timsah, the lake with salinity stratification along the Suez Canal. Saudi J Biol Sci 25(7):1247–1256
Gomes TA, Elias WP, Scaletsky IC, Guth BE, Rodrigues JF, Piazza RM et al (2016) Diarrheagenic Escherichia coli. Braz. J Microbiol 47(Suppl 1):3–30
Jang J, Hur HG, Sadowsky MJ, Byappanahalli MN, Yan T, Ishii S (2017) Environmental Escherichia coli: ecology and public health implications-a review. J Appl Microbiol 123(3):570–581
Kamala K, Rajeshkumar S, Sivaperumal P (2022) The predominance of Shiga toxin-producing E. coli in the Southeast Coast of India. Mar Pollut Bull 174:113188
Kim JS, Lee MS, Kim JH (2020) Recent Updates on outbreaks of shiga toxin-producing escherichia coli and its potential reservoirs. Front Cell Infect Microbiol 10:273
Kok T, Worswich D, Gowans E. 1996 Some serological techniques for microbial and viral infections. Practical Medical Microbiology. Collee J, Fraser A, Marmion B, Simmons A (eds), 14th ed, Edinburgh, Churchill Livingstone, UK: 179–204.
Luna-Gierke RE, Griffin PM, Gould LH, Herman K, Bopp CA, Strockbine N et al (2014) Outbreaks of non-O157 Shiga toxin-producing Escherichia coli infection: USA. Epidemiol Infect 142(11):2270–2280
Martin CC, Svanevik CS, Lunestad BT, Sekse C, Johannessen GS (2019) Isolation and characterisation of Shiga toxin-producing Escherichia coli from Norwegian bivalves. Food Microbiol 84:103268
Melton-Celsa AR (2014) Shiga toxin (Stx) classification, structure, and function. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.EHEC-0024-2013
Meng J, LeJeune JT, Zhao T, Doyle MP (2012) Enterohemorrhagic Escherichia coli. Food microbiology. Fundam Front 28:287–309
Odumosu BT, Obeten HI, Bamidele TA (2021) Incidence of multidrug-resistant Escherichia coli harbouring blaTEM and tetA genes isolated from seafoods in lagos Nigeria. Curr Microbiol 78(6):2414–2419
Prakasan S, Prabhakar P, Lekshmi M, Kumar S, Nayak BB (2018) Isolation of Shiga toxin-producing Escherichia coli harboring variant Shiga toxin genes from seafood. Vet World 11(3):379–385
Singh AS, Nayak BB, Kumar SH (2020) High Prevalence of multiple antibiotic-resistant, extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli in fresh seafood sold in retail markets of Mumbai India. Vet Sci 7(2):46
Smith JL, Fratamico PM, Gunther NWt. (2014) Shiga toxin-producing Escherichia coli. Adv Appl Microbiol 86:145–197
Steiner TS (2016) New insights into shiga toxigenic Escherichia coli pathogenesis: when less is more. J Infect Dis 213(8):1214–1215
Sujatha K, Senthilkumaar P, Sangeetha S, Gopalakrishnan M (2011) Isolation of human pathogenic bacteria in two edible fishes, Priacanthus hamrur and Megalaspis cordyla at Royapuram waters of Chennai, India. Indian J Sci Technol 4(5):539–541
Thampuran N, Surendraraj A, Surendran PK (2005) Prevalence and characterization of typical and atypical Escherichia coli from fish sold at retail in Cochin. India J Food Prot 68(10):2208–2211
Walker DI, Fok BCT, Ford CL (2020) A qPCR-MPN method for rapid quantification of Escherichia coli in bivalve molluscan shellfish. J Microbiol Methods 178:106067
Acknowledgements
Authors are very grateful to fishermen in the study area for their cooperation in sample collection.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DA, AY, HE, GA, and AA: The first draft of the manuscript was written by DA, GA, and HE. HE and AA: wrote the main manuscript text. HE: prepared figures and interpret the data. AA: reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al Qabili, D.M.A., Aboueisha, AK.M., Ibrahim, G.A. et al. Virulence and antimicrobial-resistance of shiga toxin-producing E. coli (STEC) Isolated from edible shellfish and its public health significance. Arch Microbiol 204, 510 (2022). https://doi.org/10.1007/s00203-022-03114-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03114-2