Abstract
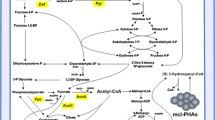
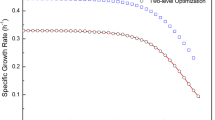
Kinetic study of growth of Pseudomonas putida mt-2 was investigated in batch culture under aerobic conditions, on glucose as initial carbon and energy source. Cell growth was continuous and three phases were found regarding accumulation of intermediates: (1) glucose was largely converted to gluconate and 2-ketogluconate, (2) then gluconate was converted to 2-ketogluconate and (3) the latter was consumed after gluconate depletion. Examination of growth kinetics and yields showed that glucose flux was mainly oriented to oxidation reduction in the periplasm and less towards biosynthesis. Values of respiratory quotient and of CO2/biomass and O2/biomass yields were characteristic of each phase. Main enzymatic activities involved in the use of these substrates were always detected meaning that concomitant assimilation is possible. However the levels of these activities varied during growth. Membrane conversions seem to have a significant energetic contribution explaining the higher specific growth rate obtained in glucose phase compared to gluconate and 2-ketogluconate ones. This is also noticeable through the evolution of the yields \( Y_{{\text{O}}_2/{\text{X}}} \) and \( Y_{{\text{CO}}_2 /{\text{X}}} \). Although the three convergent pathways are operational and can be genetically controlled, the progression of the culture in successive phases highlights an overall level of regulation in response to the energetic needs.





Similar content being viewed by others
Abbreviations
- μ :
-
Specific growth rate (h−1)
- CPR:
-
CO2 production rate (mmole l−1 h−1)
- Gn:
-
Gluconate
- KGn:
-
2-ketogluconate
- net S:
-
Net assimilated phase substrate not used in membrane oxidations (C-mole)
- OUR:
-
Oxygen uptake rate (mmole l−1 h−1)
- \( q_{{\text{CO}}_2 } \) :
-
Specific CO2 production rate (mmole g−1 h−1)
- \( q_{{\text{O}}_2 } \) :
-
Specific oxygen uptake rate (mmole g−1 h−1)
- RQ:
-
Respiratory quotient (CPR/OUR)
- S:
-
Growth phase substrate (i.e. glucose, gluconate or 2-ketogluconate)
- \( Y_{{\text{CO}}_2 /{\text{S}}} \) :
-
Yield, in percent of C-mole of accumulated CO2 per C-mole of consumed substrate
- \( Y_{{\text{CO}}_2 /{\text{X}}} \) :
-
Molar yield of produced CO2 to formed biomass (mole/mole)
- Y Gn/S :
-
Yield, in percent of C-mole of accumulated gluconate per C-mole of consumed substrate
- Y KGn/S :
-
Yield, in percent of C-mole of accumulated 2-ketogluconate per C-mole of consumed substrate
- \( Y_{{\text{O}}_2 /{\text{X}}} \) :
-
Molar yield of consumed oxygen to formed biomass (mole/mole)
- Y X/net S :
-
Net conversion yield, in percent of C-mole of formed biomass per C-mole of net assimilated phase substrate
- Y X/S :
-
Yield, in percent of C-mole of formed biomass per C-mole of utilized substrate
References
Assinder SJ, Williams PA (1990) The TOL plasmids: determinants of the catabolism of toluene and the xylenes. Adv Microb Physiol 31:1–69
Basu A, Phale PS (2006) Inducible uptake and metabolism of glucose by the phosphorylative pathway in Pseudomonas putida CSV86. FEMS Microbiol Lett 259:311–316
Conway T (1992) The Entner–Doudoroff pathway: history, physiology and molecular biology. FEMS Microbiol Rev 9:1–27
del Castillo T, Duque E, Ramos JL (2008) A set of activators and repressors control peripheral glucose pathways in Pseudomonas putida to yield a common central intermediate. J Bacteriol 190:2331–2339
del Castillo T, Ramos JL, Rodriguez-Herva JJ, Fuhrer T, Sauer U, Duque E (2007) Convergent peripheral pathways catalyze initial glucose catabolism in Pseudomonas putida: genomic and flux analysis. J Bacteriol 189:5142–5152
Dos Santos VA, Heim S, Moore ER, Stratz M, Timmis KN (2004) Insights into the genomic basis of niche specificity of Pseudomonas putida KT2440. Environ Microbiol 6:1264–1286
Eisenberg RC, Butters SJ, Quay SC, Friedman SB (1974) Glucose uptake and phosphorylation in Pseudomonas fluorescens. J Bacteriol 120:147–153
Fuhrer T, Fischer E, Sauer U (2005) Experimental identification and quantification of glucose metabolism in seven bacterial species. J Bacteriol 187:1581–1590
Greated A, Lambertsen L, Williams PA, Thomas CM (2002) Complete sequence of the IncP-9 TOL plasmid pWW0 from Pseudomonas putida. Environ Microbiol 4:856–871
Gwose I, Taraz K (1992) [Pyoverdins from Pseudomonas putida]. Z Naturforsch [C] 47:487–502
Hunt JC, Phibbs PV Jr (1983) Regulation of alternate peripheral pathways of glucose catabolism during aerobic and anaerobic growth of Pseudomonas aeruginosa. J Bacteriol 154:793–802
Jimenez JI, Minambres B, Garcia JL, Diaz E (2002) Genomic analysis of the aromatic catabolic pathways from Pseudomonas putida KT2440. Environ Microbiol 4:824–841
Kim GJ, Lee IY, Choi DK, Yoon SC, Park YH (1996) High cell density cultivation of Pseudomonas putida BM01 using glucose. J Microbiol Biotechnol 6:221–224
Kunz DA, Chapman PJ (1981) Catabolism of pseudocumene and 3-ethyltoluene by Pseudomonas putida (arvilla) mt-2: evidence for new functions of the TOL (pWWO) plasmid. J Bacteriol 146:179–191
Latour X, Lemanceau P (1997) Métabolisme carboné et énergetique des Pseudomonas ssp fluorescents saprophytes à oxydase positive. Agronomy 17:427–443
Lessie T, Neidhardt FC (1967) Adenosine triphosphate-linked control of Pseudomonas aeruginosa glucose-6-phosphate dehydrogenase. J Bacteriol 93:1337–1345
Lessie TG, Berka T, Zamanigian S (1979) Pseudomonas cepacia mutants blocked in the direct oxidative pathway of glucose degradation. J Bacteriol 139:323–325
Lessie TG, Phibbs PV Jr (1984) Alternative pathways of carbohydrate utilization in Pseudomonads. Annu Rev Microbiol 38:359–388
Ley JD (1966) 2-ketogluconic acid reductase. Meth Enzymol 9:196–200
Matsushita K, Ameyama M (1982) D-Glucose dehydrogenase from Pseudomonas fluorescens, membrane-bound. Meth Enzymol 89:149–154
Matsushita K, Shinagawa E, Adachi O, Ameyama M (1979) Membrane-bound D-gluconate dehydrogenase from Pseudomonas aeruginosa. Purification and structure of cytochrome-binding form. J Biochem (Tokyo) 85:1173–1181
Midgley M, Dawes EA (1973) The regulation of transport of glucose and methyl alpha-glucoside in Pseudomonas aeruginosa. Biochem J 132:141–154
Nandadasa HG, Andreesen M, Schlegel HG (1974) The utilization of 2-ketogluconate by Hydrogenomonas eutropha H 16. Arch Microbiol 99:15–23
Palleroni NJ (2003) Prokaryote taxonomy of the 20th century and the impact of studies on the genus Pseudomonas: a personal view. Microbiology 149:1–7
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356
Phibbs PV Jr, Eagon RG (1970) Transport and phosphorylation of glucose, fructose, and mannitol by Pseudomonas aeruginosa. Arch Biochem Biophys 138:470–482
Phibbs PV Jr, Feary TW, Blevins WT (1974) Pyruvate carboxylase deficiency in pleiotropic carbohydrate-negative mutant strains of Pseudomonas aeruginosa. J Bacteriol 118:999–1009
Ramos JL, Marques S, Timmis KN (1997) Transcriptional control of the pseudomonas TOL plasmid catabolic operons is achieved through an interplay of host factors and plasmid-encoded regulators. Annu Rev Microbiol 51:341–373
Read RR, Costerton JW (1987) Purification and characterization of adhesive exopolysaccharides from Pseudomonas putida and Pseudomonas fluorescens. Can J Microbiol 33:1080–1090
Roberts BK, Midgley M, Dawes EA (1973) The metabolism of 2-oxogluconate by Pseudomonas aeruginosa. J Gen Microbiol 78:319–329
Simons JA, Teixeira de Mattos MJ, Neijssel OM (1991) Aerobic 2-ketogluconate metabolism of Klebsiella pneumoniae NCTC 418 grown in chemostat culture. J Gen Microbiol 137:1479–1483
Sun Z, Ramsay JA, Guay M, Ramsay BA (2006) Automated feeding strategies for high-cell-density fed-batch cultivation of Pseudomonas putida KT2440. Appl Microbiol Biotechnol 71:423–431
Timmis KN (2002) Pseudomonas putida: a cosmopolitan opportunist par excellence. Environ Microbiol 4:779–781
Timmis KN, Pieper DH (1999) Bacteria designed for bioremediation. Trends Biotechnol 17:200–204
van Schie BJ et al (1985) Energy transduction by electron transfer via a pyrrolo-quinoline quinone-dependent glucose dehydrogenase in Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter calcoaceticus (var. lwoffi). J Bacteriol 163:493–499
Vicente M, Canovas JL (1973a) Glucolysis in Pseudomonas putida: physiological role of alternative routes from the analysis of defective mutants. J Bacteriol 116:908–914
Vicente M, Canovas JL (1973b) Regulation of the glucolytic enzymes in Pseudomonas putida. Arch Mikrobiol 93:53–64
Whiting PH, Midgley M, Dawes EA (1976) The role of glucose limitation in the regulation of the transport of glucose, gluconate and 2-oxogluconate, and of glucose metabolism in Pseudomonas aeruginosa. J Gen Microbiol 92:304–310
Williams PA, Murray K (1974) Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol 120:416–423
Acknowledgments
We thank Margaret Lemarié and Benoit Basset for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stuart Ferguson.
Rights and permissions
About this article
Cite this article
Latrach Tlemçani, L., Corroler, D., Barillier, D. et al. Physiological states and energetic adaptation during growth of Pseudomonas putida mt-2 on glucose. Arch Microbiol 190, 141–150 (2008). https://doi.org/10.1007/s00203-008-0380-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-008-0380-8