Abstract
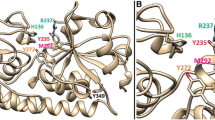
The aim of this study was to broaden the current knowledge about the Porphyromonas gingivalis heme receptor HmuR. Site-directed mutagenesis was employed to replace Glu427, Glu448, Glu458 and Glu503 by alanines and to construct a triple Glu427Ala/Glu448Ala/Glu 458Ala mutant. All iron/heme-starved P. gingivalis mutants showed decreased growth recovery when human serum as the iron/heme source was used, hmuR::ermF, hmuR E503A and hmuR E427A,E448A,E458A mutant strains being the most affected. E. coli cells expressing HmuR with mutated glutamate residues bound hemin, hemoglobin and hemin–serum albumin complex with the same efficiency as did the wild-type recombinant protein, suggesting that the residues were not directly involved in heme binding. These data indicate that in addition to two conserved histidine residues (His95 and His434), NPDL and YRAP motifs, conserved glutamate residues are important for HmuR to utilize heme present in serum hemoproteins.



Similar content being viewed by others
References
Bairoch A, Boeekmann B (1991) The SWISS-PROT protein sequence data bank. Nucleic Acids Res 19:2247–2249
Boulton IC, Yost MK, Anderson JE, Cornelissen CN (2000) Identification of discrete domains within gonococcal transferrin-binding protein A that are necessary for ligand binding and iron uptake functions. Infect Immun 68:6988–6996
Bracken CS, Baer MT, Abdur-Rashid A, Helms W, Stojiljkovic I (1999) Use of heme-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J Bacteriol 181:6063–6072
Braun V (1995) Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev 16:295–307
Buchanan SK, Smith BS, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J (1999) Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol 6:56–63
Chakraborty R, Lemke EA, Cao Z, Klebba PE, Van der Helm D (2003) Identification and mutational studies of conserved amino acids in the outer membrane receptor protein, FepA, which affect transport but not binding of ferric–enterobactin in Escherichia coli. Biometals 16:507–518
Chimento DP, Mohanty AK, Kadner RJ, Wiener MC (2003) Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat Struct Biol 10:394–401
Cobessi D, Celia H, Folschweiller N, Schalk IJ, Abdallah MA, Pattus F (2005a) The crystal structure of the pyoverdine outer membrane receptor FpvA from Pseudomonas aeruginosa at 3.6 Å resolution. J Mol Biol 347:121–134
Cobessi D, Celia H, Pattus F (2005b) Crystal structure at high resolution of ferric-pyochelin and its membrane receptor FptA from Pseudomonas aeruginosa. J Mol Biol 352:893–904
Cope LD, Thomas SE, Hrkal Z, Hansen EJ (1998) Binding of heme–hemopexin complexes by soluble HxuA protein allows utilization of this complexed heme by Haemophilus influenzae. Infect Immun 66:4511–4516
Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W (1998) Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215–2220
Ferguson AD, Braun V, Fiedler HP, Coulton JW, Diederichs K, Welte W (2000) Crystal structure of the antibiotic albomycin in complex with the outer membrane transporter FhuA. Protein Sci 9:956–963
Frangipane ME, Morton DJ, Wooten JA, Pozsgay JM, Stull TL (1994) Binding of human hemoglobin by Haemophilus influenzae. FEMS Microbiol Lett 118:243–248
Genco CA, Dixon DW (2001) Emerging strategies in microbial heme capture. Mol Microbiol 391:1–11
Holt SC, Kesavalu L, Walker S, Genco CA (1999) Virulence factors of Porphyromonas gingivalis. Periodontol 2000 20:168–238
James CE, Hasegawa Y, Park Y, Yeung V, Tribble GD, Kuboniwa M, Demuth DR, Lamont RJ (2006) LuxS involvement in the regulation of genes coding for hemin and iron acquisition systems in Porphyromonas gingivalis. Infect Immun 74:3834–3844
Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci 23:403–405
Kusaba A, Ansai T, Akifusa S, Nakahigashi K, Taketani S, Inokuchi H, Takehara T (2002) Cloning and expression of a Porphyromonas gingivalis gene for protoporphyrinogen oxidase by complementation of a hemG mutant of Escherichia coli. Oral Microbiol Immunol 17:290–295
Liu X, Sroka A, Potempa J, Genco CA (2004) Coordinate expression of the Porphyromonas gingivalis lysine-specific gingipain proteinase, Kgp, arginine-specific gingipain proteinase, RgpA, and the heme/hemoglobin receptor, HmuR. Biol Chem 385:1049–1057
Liu X, Olczak T, Guo HC, Dixon DW, Genco CA (2006) Identification of amino acid residues involved in heme binding and hemoprotein utilization in the Porphyromonas gingivalis heme receptor HmuR. Infect Immun 74:1222–1232
Locher KP, Rees B, Koebnik R, Mitschler A, Moulinier L, Rosenbush JP, Moras D (1998) Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771–778
Nelson KE, Fleischmann RD, DeBoy RT, Paulsen IT, Fouts DE, Eisen JA, Daugherty SC, Dodson RJ, Durkin AS, Gwinn M, Haft DH, Kolonay JF, Nelson WC, Mason T, Tallon L, Gray J, Granger D, Tettelin H, Dong H, Galvin JL, Duncan MJ, Dewhirst FE, Fraser CM (2003) Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J Bacteriol 185:5591–5601
Olczak T, Dixon DW, Genco CA (2001) Binding specificity of the Porphyromonas gingivalis heme and hemoglobin receptor HmuR, gingipain K, and gingipain R1 for heme, porphyrins, and metalloporphyrins. J Bacteriol 183:5599–5608
Olczak T, Simpson W, Liu X, Genco CA (2005) Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol Rev 29:119–144
Pawelek PD, Coulton JW (2004) Hemoglobin-binding protein HgbA in the outer membrane of Actinobacillus pleuropneumoniae: homology modelling reveals regions of potential interactions with hemoglobin and heme. J Mol Graph Model 23:211–221
Perkins-Balding D, Baer MT, Stojiljkovic I (2003) Identification of functionally important regions of a haemoglobin receptor from Neisseria meningitidis. Microbiology 149:3423–3435
Pihlstrom BL, Michalowicz BS, Johnson NW (2005) Periodontal diseases. Lancet 366:1809–1820
Postle K, Kadner RJ (2003) Touch and go: tying TonB to transport. Mol Microbiol 49:869–882
Potempa J, Sroka A, Imamura T, Travis J (2003) Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci 4:397–407
Roper JM, Raux E, Brindley AA, Schubert HL, Gharbia SE, Shah HN, Warren MJ (2000) The enigma of cobalamin (vitamin B12) biosynthesis in Porphyromonas gingivalis. Identification and characterization of a functional corrin pathway. J Biol Chem 275:40316–40323
Simpson W, Wang CY, Bond V, Potempa J, Mikolajczyk-Pawlinska J, Travis J, Genco CA (1999) Transposition of the endogenous insertion sequence element IS1126 modulates gingipain expression in Porphyromonas gingivalis. Infect Immun 67:5012–5020
Simpson W, Olczak T, Genco CA (2000) Characterization and expression of HmuR, a TonB-dependent hemoglobin receptor of Porphyromonas gingivalis. J Bacteriol 182:5737–5748
Simpson W, Olczak T, Genco CA (2004) Lysine-specific gingipain K and heme/hemoglobin receptor HmuR are involved in heme utilization in Porphyromonas gingivalis. Acta Biochim Pol 51:253–262
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Thompson JM, Jones HA, Perry RD (1999) Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization. Infect Immun 67:3879–3892
Xu X, Kolodrubetz D (2002) Construction and analysis of hemin binding protein mutants in the oral pathogen Treponema denticola. Res Microbiol 153:569–577
Yost-Daljev MK, Cornelissen CN (2004) Determination of surface-exposed, functional domains of gonococcal transferrin-binding protein A. Infect Immun 72:1775–1785
Acknowledgments
Dr. C. A. Genco (Department of Medicine, Section of Infectious Diseases, Boston University School of Medicine, USA) is gratefully acknowledged for giving T.O., the opportunity to continue studies on P. gingivalis heme utilization. The author thanks Dr. X. Liu (Department of Medicine, Section of Infectious Diseases, Boston University School of Medicine, USA) for localization of glutamate residues in the theoretical HmuR model published previously (Olczak et al. 2005; Liu et al. 2006). This work was supported in part by grant No. 3 P05A 113 24 from the Department of Scientific Research of Ministry of Science and Higher Education, Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olczak, T. Analysis of conserved glutamate residues in Porphyromonas gingivalis outer membrane receptor HmuR: toward a further understanding of heme uptake. Arch Microbiol 186, 393–402 (2006). https://doi.org/10.1007/s00203-006-0151-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-006-0151-3