Abstract
Summary
In women with osteoporosis treated with alendronate for >12 months and oral bisphosphonates for >3 of the last 4 years, switching to MK-5442, a calcium receptor antagonist, stimulated endogenous parathyroid hormone (PTH) secretion and increased bone turnover marker levels, but produced a decline in bone mineral density (BMD) at all sites.
Introduction
This study assessed the effects of switching from long-term oral bisphosphonate therapy to the calcium-sensing receptor antagonist MK-5442 on BMD and bone turnover markers (BTMs) in post-menopausal women with osteoporosis.
Methods
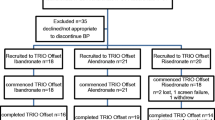
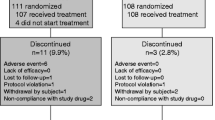
This randomized, active and placebo-controlled, dose-ranging study enrolled 526 postmenopausal women, who had taken alendronate (ALN) for ≥12 months preceding the trial and any oral bisphosphonate for ≥3 of the preceding 4 years and had spine or hip BMD T-scores ≤−2.5 or ≤−1.5 with ≥1 prior fragility fracture. Women were randomized to continue ALN 70 mg weekly or switch to MK-5442 (5, 7.5, 10, or 15 mg daily) or placebo.
Results
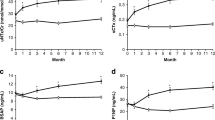
Switching from ALN to MK-5442 produced a dose-dependent parathyroid hormone (PTH) pulse of threefold to sixfold above baseline at 1 h, with PTH levels that remained twofold to threefold above baseline at 4 h and returned to baseline by 24 h. Switching to MK-5442 or placebo increased BTM levels compared to baseline within 3 months and MK-5442 10 mg increased BTM levels compared to placebo by 6 months. With all MK-5442 doses and placebo, spine and hip BMD declined from baseline, and at 12 months, BMD levels were below those who continued ALN (all groups P < 0.05 vs ALN). There was also a dose-dependent increase in the incidence of hypercalcemia with MK-5442.
Conclusion
Switching from ALN to MK-5442 resulted in a pulsatile increase in PTH and increases in BTMs, but a decline in BMD compared with continued ALN. MK-5442 is not a viable option for the treatment of osteoporosis.



Similar content being viewed by others
References
Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 85:4118–24
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–38
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–99
McClung M, Recker R, Miller P, Fiske D, Minkoff J, Kriegman A, Zhou W, Adera M, Davis J (2007) Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone 41:122–28
Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25:72–81
Matheny JB, Slyfield CR, Tkachenko EV, Lin I, Ehlert KM, Tomlinson RE, Wilson DL, Hernandez CJ (2013) Anti-resorptive agents reduce the size of resorption cavities: a three-dimensional dynamic bone histomorphometry study. Bone 57(1):277–83
Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K (2001) Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone 29(2):185–91
Seeman E (2007) Is a change in bone mineral density a sensitive and specific surrogate of anti-fracture efficacy? Bone 41(1):308–17
Boonen S, Marin F, Obermayer-Pietsch B, Simoes ME, Barker C, Glass EV, Hadji P, Lyritis G, Oertel H, Nickelsen T, McCloskey EV (2008) Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 93(1):852–60
Cosman F, Wermers RA, Recknor C, Mauck KF, Xie L, Glass EV, Krege JH (2009) Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab 94:3772–80
Cosman F, Keaveny TM, Kopperdahl D, Wermers RA, Wan X, Krohn KD, Krege JH (2013) Hip and spine strength effects of adding versus switching to teriparatide in postmenopausal women with osteoporosis treated with prior alendronate or raloxifene. J Bone Miner Res 28:1328–36
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19(2):745–51
Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, Saag K, Greenspan SL, Seeman E, Boonen S, Meeves S, Lang TF, Bilezikian JP (2008) Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 93(1):3785–93
Cosman F (2014) Anabolic and Antiresorptive Therapy for Osteoporosis: Combination and Sequential Approaches. Curr Osteoporos Rep 12:385–95
Eli Lilly and Co. FORTEO (teriparatide). Prescribing Information. 2012; 1-13. http://www.pi.lilly.com/us/forteo-pi.pdf Accessed 05 November 2015.
Tashjian AH Jr, Gagel RF (2006) Teriparatide [human PTH(1–34)]: 2.5 years of experience on the use and safety of the drug for the treatment of osteoporosis. J Bone Miner Res 21:354–65
Canalis E, Giustina A, Bilezikian JP (2007) Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 357:905–16
Cosman F, Lane NE, Bolognese MA, Zanchetta JR, Garcia-Hernandez PA, Sees K, Matriano JA, Gaumer K, Daddona PE (2010) Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab 95:151–58
Fitzpatrick LA, Dabrowski CE, Cicconetti G, Gordon DN, Papapoulos S, Bone HG III, Bilezikian JP (2011) The effects of ronacaleret, a calcium-sensing receptor antagonist, on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mineral density. J Clin Endocrinol Metab 96:2441–49
Fukumoto S, Nakamura T, Nishizawa Y, Hayashi M, Matsumoto T (2009) Randomized, single-blinded placebo-controlled study of a novel calcilytic, JTT-305, in patients with postmenopausal osteoporosis. J Bone Miner Res 24 (Supp1 1)
Liang KY, Zeger SL (2000) Longitudinal data analysis of continuous and discrete responses for pre-post designs. Indian J Stat 62:134–48
Halse J, Greenspan S, Cosman F, Ellis G, Santora A, Leung A, Heyden N, Samanta S, Doleckyj S, Rosenberg E, Denker AE (2014) A phase 2, randomized, placebo-controlled, dose-ranging study of the calcium-sensing receptor antagonist MK-5442 in the treatment of postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 99:E2207-15.
Halse J, Greenspan S, Cosman F, Ellis G, Rosenberg E, Heyden N DS, Samanta S, Weili H, Santora A, Leung A, Denker A (2012) A phase 2b, randomized, placebo-controlled, dose-ranging study of MK-5442 in the treatment of postmenopausal women with osteoporosis. J Bone Miner Res 27 (Suppl 1)
Ryder KM, Tanner SB, Carbone L, Williams JE, Taylor HM, Bush A, Pintea V, Watsky MA (2010) Teriparatide is safe and effectively increases bone biomarkers in institutionalized individuals with osteoporosis. J Bone Miner Metab 28:233–39
Anastasilakis AD, Goulis DG, Polyzos SA, Gerou S, Koukoulis GN, Efstathiadou Z, Kita M, Avramidis A (2008) Head-to-head comparison of risedronate vs. teriparatide on bone turnover markers in women with postmenopausal osteoporosis: a randomised trial. Int J Clin Pract 62:919–24
Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege JH (2011) PINP as an aid for monitoring patients treated with teriparatide. Bone 48:798–803
Satterwhite J, Heathman M, Miller PD, Marin F, Glass EV, Dobnig H (2010) Pharmacokinetics of teriparatide (rhPTH[1–34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcif Tissue Int 87:485–92
Tam CS, Heersche JN, Murray TM, Parsons JA (1982) Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology 110:506–12
Hock JM, Gera I (1992) Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res 7:65–72
Iida-Klein A, Lu SS, Kapadia R, Burkhart M, Moreno A, Dempster DW, Lindsay R (2005) Short-term continuous infusion of human parathyroid hormone 1–34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J Endocrinol 186:549–57
Lewiecki EM, Miller PD (2013) Skeletal effects of primary hyperparathyroidism: bone mineral density and fracture risk. J Clin Densitom 16:28–32
Hansen S, Hauge EM, Beck Jensen JE, Brixen K (2013) Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res 28:736–45
Brown EM, Pollak M, Hebert SC (1995) Molecular mechanisms underlying the sensing of extracellular Ca2+ by parathyroid and kidney cells. Eur J Endocrinol 132(1):523–31
Brown EM, MacLeod RJ (2001) Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81:239–97
Marie PJ (2010) The calcium-sensing receptor in bone cells: a potential therapeutic target in osteoporosis. Bone 46:571–76
Xue Y, Xiao Y, Liu J, Karaplis AC, Pollak MR, Brown EM, Miao D, Goltzman D (2012) The calcium-sensing receptor complements parathyroid hormone-induced bone turnover in discrete skeletal compartments in mice. Am J Physiol Endocrinol Metab 302:E841–E851
Acknowledgments
The authors would like to thank Boyd B Scott, Ph.D., and Jennifer Pawlowski of Merck & Co. Inc. for their assistance in the preparation and submission of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
FC has received consulting, advisory board, and speaker’s bureau fees or honorarium from the study sponsor, Merck & Co. Inc. FC has received consulting, advisory board, and speaker’s bureau fees or honorarium from Amgen, Eli Lilly, Novartis, GSK, Tarsa, Zosano, Pfizer, Unigene, Asahi-Kasei, and Enteris. FC’s Institution has received grants from Merck, Amgen, Eli Lilly, and Novartis.
NG has received consultancy fees and speaker’s bureau fees from GGM.
MM has received consulting, advisory board, and honorarium from the study sponsor, Merck & Co. Inc. MM has received consulting, advisory board, and honorarium from Amgen and Eli Lilly.
JF has received speaker’s bureau fees or honorarium from Merck & Co. Inc. Institution has received grants from the study sponsor, Merck & Co. Inc.
TDV has received advisory board, travel support, and speaker’s bureau fees or honorarium from the study sponsor, Merck & Co. Inc. TDV has received advisory board, travel support, and speaker’s bureau fees or honorarium from Adcock Ingram, Servier, Amgen, and Pfizer.
Authors AD, NH, AL, ER, SS, JM, and AC are all employees of Merck & Co. Inc and may own stock or stock options.
Funding
This study was funded by Merck & Co. Inc. The study protocol was the responsibility of Merck & Co. Inc. and designed in collaboration with the steering committee composed of the academic authors and various health authorities. All analyses were conducted by scientists at Merck & Co. Inc. All authors had full access to the study data and vouch for the completeness of the data set and the performance of the data analyses. This study was registered with clinicaltrials.gov identifier: with registration number NCT00996801.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1.52 mb)
Rights and permissions
About this article
Cite this article
Cosman, F., Gilchrist, N., McClung, M. et al. A phase 2 study of MK-5442, a calcium-sensing receptor antagonist, in postmenopausal women with osteoporosis after long-term use of oral bisphosphonates. Osteoporos Int 27, 377–386 (2016). https://doi.org/10.1007/s00198-015-3392-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3392-7