Abstract
Summary
The study estimates the cost of poor and suboptimal refill compliance by estimating fracture costs and assessing the association between refill compliance with oral bisphosphonates and incident fractures using Danish health registers. Patients with poor and suboptimal refill compliance had more major osteoporotic fractures, and the direct costs related to hospital care, primary care, and pharmaceutical treatment for these excess fractures reached almost 14 M DKK (2.5 M USD) for the study population which compares to a national annual excess cost of around 17 M DKK (3.1 M USD) using 2011 prescription prevalence.
Introduction
Adherence to oral anti-osteoporosis treatment has been shown in several studies to be relatively low and the potential impact on fracture burden is high. The aim of the study was to assess the association between refill compliance and all-cause health care costs.
Methods
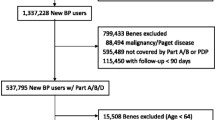
A national dataset was extracted with all treatment-naive patients who began oral bisphosphonate (BP) treatment for osteoporosis in Denmark between 1997 and 2006 (N = 54,876, 87 % women). Patients who survived for at least 2 years (N = 47,176) were divided into groups based on Medication Possession Ratio (MPR). Logistic regressions were used to derive difference in the probability of incident fractures between the three MPR groups. Fracture costs (related to medication use, primary care practice, specialists, and hospitals) were derived by comparing cost 12 months before and after fracture.
Results
For alendronate, the adjusted risk of major osteoporotic fractures was significantly reduced (OR 0.768; 0.686–0.859), including fractures of the hip (0.718; 0.609–0.846) and humerus (0.54; 0.431–0.677) with MPR ≥ 0.8. The risk reduction was lower with etidronate. Over 2 years, a total of 171 hip fractures and 53 other major osteoporotic fractures were attributed to suboptimal or poor refill compliance, with an excess cost of 13.7 M DKK (2.5 M USD).
Conclusions
Poor refill compliance is not unusual in patients on oral bisphosphonates, and we demonstrate that this is accompanied by excess major osteoporotic fractures and health care costs at the societal level.



Similar content being viewed by others
References
Eastell R, Walsh JS, Watts NB, Siris E (2011) Bisphosphonates for postmenopausal osteoporosis. Bone 49(1):82–88
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361(8):756–765
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY et al (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344(19):1434–1441
Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD et al (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. Obstet Gynecol Surv 59(7):526–527
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637–645
Cramer JA, Gold DT, Silverman SL, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18(8):1023–3
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21:1453–1460
Jones TJ, Petrella RJ, Crilly R (2008) Determinants of persistence with weekly bisphosphonates in patients with osteoporosis. J Rheumatol 35:1865–1873
Cotté F-E, De Pouvourville G (2011) Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC health services research. BioMed Central Ltd 11(1):151
Ziller V, Kostev K, Kyvernitakis I, Boeckhoff J, Hadji P (2012) Persistence and compliance of medications used in the treatment of osteoporosis analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther 50(5):315–322
Silverman SL, Gold DT, Cramer JA (2007) Reduced fracture rates observed only in patients with proper persistence and compliance with bisphosphonate therapies. South Med J 100:1214–1218
Seeman E, Compston J, Adachi J, Brandi ML, Cooper C, Dawson-Hughes B et al (2007) Non-compliance: the Achilles' heel of anti-fracture efficacy. Osteoporos Int 18:711–719
Brask-Rasmussen D, Cadarette SM, Eskildsen P, Abrahamsen B (2011) Osteoporosis therapy following bone densitometry—patient expectations as determinants of drug initiation and persistence. Osteoporos Int 22(5):1493–1501
Block AE, Solomon DH, Cadarette SM, Mogun H, Choudhry NK (2008) Patient and physician predictors of post-fracture osteoporosis management. J Gen Intern Med. 23(9):1447–51
McHorney CA, Schousboe JT, Cline RR, Weiss TW (2008) The impact of osteoporosis medication beliefs and side-effect experiences on non-adherence to oral bisphosphonates. Curr Med Res Opin. 23(12):3137–52
Hansen, Carrinna; Pedersen, Birthe D; Konradsen, Hanne; Abrahamsen B (2012) Anti-osteoporotic therapy in denmark—predictors and demographics of poor refill compliance and poor persistence. Osteoporosis Int. (in press)
Ross S, Samuels E, Gairy K, Iqbal S, Badamgarav E, Siris E (2011) A meta-analysis of osteoporotic fracture risk with medication. JVAL Elsevier Inc 14(4):571–581
Huybrechts KF, Ishak KJ, Caro JJ (2006) Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 38:922–928
Cotté F-E, Mercier F, De Pouvourville G (2008) Relationship between compliance and persistence with osteoporosis medications and fracture risk in primary health care in France: a retrospective case–control analysis. Clin Ther 30(12):2410–2422
Orsini LS, Rousculp MD, Long SR, Wang S (2005) Health care utilization and expenditures in the United States: a study of osteoporosis-related fractures. Osteoporos Int 16(4):359–371
Halpern R, Becker L, Iqbal SU, Kazis LE, Macarios D, Badamgarav E (2011) The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manage Care Pharm: JMCP 17(1):25–39
Sheehy O, Kindundu C, Barbeau M, LeLorier J (2009) Adherence to weekly oral bisphosphonate therapy: cost of wasted drugs and fractures. Osteoporos Int 20(9):1583–1594
Nielsen DS, Langdahl BL, Sørensen OH, Sørensen HA, Brixen KT (2010) Persistence to medical treatment of osteoporosis in women at three different clinical settings—a historical cohort study. Scand J Public Health 38(5):502–507
Roerholt C, Eiken P, Abrahamsen B (2009) Initiation of anti-osteoporotic therapy in patients with recent fractures: a nationwide analysis of prescription rates and persistence. Osteoporos Int 20(2):299–307
Kilgore ML, Curtis JR, Delzell E, Becker DJ, Arora T, Saag KG, et al. (2012) A close examination of health care expenditures related to fractures. J Bone Mineral Res. 28(4):816–20
Khan MA, Hossain FS, Dashti Z, Muthukumar N (2012) Causes and predictors of early re-admission after surgery for a fracture of the hip. J Bone Joint Surg Br 94(5):690–697
Zethraeus N, Gerdtham UG (1998) Estimating the costs of hip fracture and potential savings. Int J of Tech Assess Health Care 14(2):255–267
Borgström F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O et al (2006) Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 17(5):637–650
Lin JH, Zhang SM, Manson JE (2011) Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prev Res (Phila) 4(9):1360–1365
Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC (2012) Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur J Clin Pharmacol 68(10):1365–1373
Pazianas M, Abrahamsen B, Eiken PA, Eastell R, Russell RGG (2012) Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate-Danish National Register Based Cohort Study. Osteoporos Int 23(11):2693–2701
Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V (2010) Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case–control analysis within a UK primary care cohort. BMJ (Clin Res Ed.). 341:c4444
Chlebowski RT, Chen Z, Cauley JA, Anderson G, Rodabough RJ, McTiernan A et al (2010) Oral bisphosphonate use and breast cancer incidence in postmenopausal women. J Clin Oncol 28(22):3582–3590
Vestergaard P, Fischer L, Mele M (2011) Use of Bisphosphonates and Risk of Breast Cancer. Breast 88(4):255–62
Pazianas M, Russell RGG (2012) Potential therapeutic effects of oral bisphosphonates on the intestine. Ann N Y Acad Sci 1240:19–25
Majumdar SR, Kim N, Colman I, Chahal AM, Raymond G, Jen H et al (2005) Incidental vertebral fractures discovered with chest radiography in the emergency department: prevalence, recognition, and osteoporosis management in a cohort of elderly patients. Arch Intern Med 165(8):905–909
Majumdar SR, Villa-Roel C, Lyons KJ, Rowe BH (2010) Prevalence and predictors of vertebral fracture in patients with chronic obstructive pulmonary disease. Respir Med 104(2):260–266
Acknowledgments
This study was funded through a research grant from Amgen (Europe) GmbH. The funding source did not have any influence on the data analysis, interpretation, or conclusions but approved the final manuscript prior to submission. KRO is an employee of GSK Denmark and of the University of Southern Denmark and participated in this work as a GSK employee.
Conflicts of interest
CH has received speaker's fees from Eli Lilly. KRO is a GSK Denmark employee. BA has served as an investigator in clinical trials and on advisory boards and/or speakers panels for pharmaceutical companies that produce osteoporosis drugs. Clinical trials: Amgen, NPS Pharmaceuticals. Advisory boards: Amgen, Takeda-Nycomed, Merck. Speakers panels: Amgen, Nycomed, Eli Lilly, Merck.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olsen, K.R., Hansen, C. & Abrahamsen, B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs—an analysis using national health databases. Osteoporos Int 24, 2639–2647 (2013). https://doi.org/10.1007/s00198-013-2365-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2365-y