Abstract
Introduction
This study aimed to examine long-term persistence in new users of oral bisphosphonates in a population-wide cohort in Manitoba, Canada.
Materials and methods
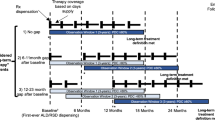
A longitudinal observational study was conducted using administrative health data characterizing long-term bisphosphonate persistence in those who started treatment between 1997 and 2018. Treatment discontinuation was evaluated using Kaplan–Meier methods. Cox regression was used to examine associations between discontinuation and osteoporosis diagnosis, previous fractures, and age. A sub-analysis of users with FRAX scores examined the relationship between 10-year fracture risk estimations and discontinuation.
Results
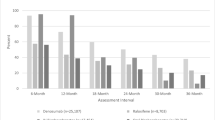
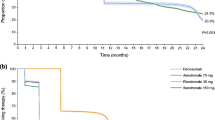
Of 42,249 new bisphosphonate users, median age was 71 years, with 88.6% being female. Median duration of bisphosphonate use was 0.95 years (IQR 0.25, 3.9 years). Overall, 47.9% of incident users persisted up to 1 year, 25.0% persisted up to 3 years, and 14.1% up to 5 years. Presence of an indication for bisphosphonate use was associated with decreased discontinuation risk. Persistence generally increased with age. Having a BMD test performed was a predictor of lower discontinuation. The strongest predictor was having an osteoporosis diagnosis [HR for discontinuation = 0.68 (95% CI 0.66, 0.70)]. In users with FRAX scores (n = 14,114), moderate-risk [HR = 0.86 (95% CI 0.77, 0.96)] and high-risk users [HR = 0.77 (95% CI 0.69, 0.85)] were less likely to discontinue compared to lower-risk users.
Conclusions
A rapid decline in bisphosphonate persistence was shown. Almost half of users would not be expected to achieve clinically relevant benefits with a persistence of less than 1 year. Allowing informed choice in high-risk patients may be the best way to focus on those likely to benefit and persist with treatment.

Similar content being viewed by others
References
Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR (2009) Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. J Am Med Assoc 301:513–521
Bessette L, Jean S, Lapointe-Garant MP, Belzile EL, Davison KS, Ste-Marie LG, Brown JP (2012) Direct medical costs attributable to peripheral fractures in Canadian postmenopausal women. Osteoporos Int 23:1757–1768
Tarride JE, Hopkins RB, Leslie WD, Morin S, Adachi JD, Papaioannou A, Bessette L, Brown JP, Goeree R (2012) The burden of illness of osteoporosis in Canada. Osteoporos Int 23:2591–2600
Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR, for the Fit Research Group (2000) Fracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial. J Clin Endocrinol Metab 85:4118–4124
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamon T, Eastell R, Meunier PJ, Reginster J-Y, for the HIP Intervention Program Study Group (2001) Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 344:333–340
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chestnut CH, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD, for the VERT Study Group (1999) Effect of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. J Am Med Assoc 282:1344–1352
Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Welch V, Coyle D, Tugwell P (2008) Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd001155.pub2(Art. No.: CD001155)
Fink HA, MacDonald R, Forte ML, Rosebush CE, Ensrud KE, Schousboe JT, Nelson VA, Ullman K, Butler M, Olson CM, Taylor BC, Brasure M, Wilt TJ (2019) Long-Term Drug Therapy and Drug Holidays for Osteoporosis Fracture Prevention: A Systematic Review. Comparative Effectiveness Review No. 218. (Prepared by the Minnesota Evidence-based Practice Center under Contract No. 290-2015-00008-I) AHRQ Publication No. 19-EHC016-EF. Rockville, MD: Agency for Healthcare Research and Quality
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R, Foundation National Osteoporosis (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381
Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, Hanley DA, Hodsman A, Jamal SA, Kaiser SM, Kvern B, Siminoski K, Leslie WD (2010) 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 182:1864–1873
Qaseem A, Forciea MA, McLean RD, Denberg TD, for the Clinical Guidelines Committee of the American College of Physicians (2017) Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med 166:818–839
Ofori-Asenso R, Ilomaki J, Tacey M, Si S, Curtis AJ, Zomer E, Bell JS, Zoungas S, Liew D (2019) Br J Clin Pharmacol 85:227–235
Ofori-Asenso R, Ilomaki J, Tacey M, Zomer E, Curtis AJ, Bell JS, Zoungas S, Liew D (2018) Patterns of statin use and long-term adherence and persistence among older adults with diabetes. J Diabetes 10:699–707
Ofori-Asenso R, Jakhu A, Zomer E, Curtis AJ, Korhonen MJ, Nelson M, Gambhir M, Tonkin A, Liew D, Zoungas S (2018) Adherence and persistence among statin users aged 65 years and over: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 73:813–819
Burden AM, Paterson JM, Gruneir A, Cadarette SM (2015) Adherence to osteoporosis pharmacotherapy is underestimated using days supply values in electronic pharmacy claims data. Pharmacoepidemiol Drug Saf 24:67–74
Sheehy O, Kindundu CM, Barbeau M, LeLorier J (2009) Differences in persistence among different weekly bisphosphonate medications. Osteoporos Int 20:1369–1376
Sheehy O, Kindundu CM, Barbeau M, LeLorier J (2009) Adherence to weekly oral bisphosphonate therapy: cost of wasted drugs and fractures. Osteoporos Int 20:1583–1594
Jones TJ, Petrella RJ, Crilly R (2008) Determinants of persistence with weekly bisphosphonates in patients with osteoporosis. J Rheumatol 35:1865–1873
Burden AM, Paterson JM, Solomon DH, Mamdani M, Juurlink DN, Cadarette SM (2012) Bisphosphonate prescribing, persistence and cumulative exposure in Ontario, Canada. Osteoporos Int 23:1075–1082
Klop C, Welsing PMJ, Elders PJM et al (2015) Long-term persistence with anti-osteoporosis drugs after fracture. Osteoporos Int 26:1831–1840
Van Boven JFM, De Boer PT, Postma MJ, Vegter S (2013) Persistence with osteoporosis medication among newly-treated osteoporotic patients. J Bone Miner Metab 31:562–570
Imaz I, Zegarra P, Gonzalez-Enriquez J, Rubio B, Alcazar R, Amate M (2010) Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int 21:1943–1951
Siris ES, Selby PL, Saag KG, Borgström F, Herings RMC, Silverman SL (2009) Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 122:S3–S13
Roos LL, Brownell M, Lix L, Roos NP, Walld R, Macwilliam L (2008) From health research to social research: privacy, methods, approaches. Soc Sci Med 66:117–129
Jutte DP, Roos LL, Brownell MD (2011) Administrative record linkage as a tool for public health research. Annu Rev Public Health 32:91–108
World Health Organization. ATC/DDD Index 2019. Oslo: WHO Collaborating Centre for Drug Statistics Methodology (updated December, 2018). https://www.whocc.no/atc_ddd_index/. Accessed 8 Oct 2019
Martens PJ, Nathan N, Forget E, Lix L, Turner D, Prior H, Walld R, Soodeen R-A, Rajotte L, Ekuma O (2015) The cost of smoking: a Manitoba study. Manitoba Centre for Health Policy, Winnipeg
van den Boogaard CHA, Breekveldt-Postma NS, Borggreve SE, Goettsch WG, Herings RMC (2006) Persistent bisphosphonate use and the risk of osteoporotic fractures in clinical practice: a database analysis study. Cur Med Res Opin 22:1757–1764. https://doi.org/10.1185/030079906X132370
FRAX. Fracture Risk Assessment Tool. Sheffield: Centre for Metabolic Bone Diseases. https://www.sheffield.ac.uk/FRAX/. Accessed 8 Oct 2019
Roerholt C, Eiken P, Abrahamsen B (2009) Initiation of anti-osteoporotic therapy in patients with recent fractures: a nationwide analysis of prescription rates and persistence. Osteoporos Int 20:299–307
Wozniak LA, Johnson JA, McAlister FA, Beaupre LA, Bellerose D, Rowe BH, Majumdar SR (2017) Understanding fragility fracture patients’ decision-making process regarding bisphosphonate treatment. Osteoporos Int 28:219–229
Goldshtein I, Rouach V, Shamir-Stein N, Yu J, Chodick G (2016) Role of side effects, physician involvement, and patient perception in non-adherence with oral bisphosphonates. Adv Ther 33:1374–1384
Yun H, Curtis JR, Guo L, Kilgore M, Muntner P, Saag K, Matthews R, Morrisey M, Wright NC, Becker DJ, Delzell E (2014) Patterns and predictors of osteoporosis medication discontinuation and switching among Medicare beneficiaries. BMC Musculoskelet Disord 15:112. https://doi.org/10.1186/1471-2474-15-112
Jarvinen TLN, Michaelsson K, Aspenberg P, Sievanen H (2015) Osteoporosis: the emperor has no clothes. J Intern Med 277:662–673
Gates BJ, Sonnett TE, DuVall CAK, Dobbins EK (2009) Review of osteoporosis pharmacotherapy for geriatric patients. Am J Geriatr Pharmacother 7:293–323
Acknowledgements
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository under project #H2016:16 (HIPC# 2016/2017-05). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, Senior and Active Living, or other data providers is intended or should be inferred. Data used in this study are from the Population Health Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba and were derived from data provided by Manitoba Health, Seniors and Active Living and the Manitoba Centre for Health Policy, University of Manitoba and were derived from data provided by Manitoba.
Funding
This study was funded by a University of Manitoba internal grant.
Author information
Authors and Affiliations
Contributions
All authors (KJF, SB, and JF) contributed to the study design, analysis of the data, and writing and review of the final manuscript. KJF provided additional analytical and statistical support.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Friesen, K.J., Bugden, S. & Falk, J. Time to benefit and the long-term persistence of new users of oral bisphosphonates. J Bone Miner Metab 38, 371–377 (2020). https://doi.org/10.1007/s00774-019-01069-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-019-01069-x