Abstract
Summary
To elucidate whether serum levels of pepsinogens are associated with the occurrence of gastrointestinal adverse events induced by amino-bisphosphonates (amino-BP), the serum levels of pepsinogen were measured in amino-BP users. Our results indicate that measurement of pepsinogen I is useful in predicting gastric distress induced by amino-BP in osteoporosis.
Introduction
To elucidate whether serum levels of pepsinogens are associated with the occurrence of gastrointestinal adverse events induced by amino-BP, the serum levels of pepsinogen I and II were measured in amino-BP users.
Methods
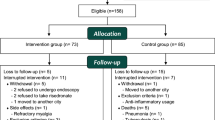
When the patients complained of gastric distress symptoms during the first 6 months after amino-BP use resulting in discontinuation of the drug, endoscopical examinations were performed to assess whether gastric lesions were present. A total of 223 amino-BP users were enrolled in the study, of which 47 patients refused to take the drug due to gastric distress symptoms. The remaining 176 patients did not complain of any gastric distress.
Results
Among 47 patients, eight patients showed obvious gastric lesions such as gastric or duodenal ulcers and acute gastric mucosal lesions in the endoscopical examination. The remaining 39 patients did not show any gastric lesions. The possible confounding factors, such as a Helicobactor pylori infection or concurrent use of ulcerogenic agents, did cause not affect gastric distress in amino-BP users. The serum pepsinogen I level was significantly associated with severity of the gastric lesion 46.8 ± 27.7, 60.8 ± 32.4, and 103.4 ± 49.2 ng/ml for patients without any gastric distress, with gastric distress accompanied no gastric lesions, and with gastric distress accompanied gastric lesions, respectively.
Conclusions
ROC analysis revealed that the cutoff value of pepsinogen I for expectation of gastric regions was 76.8 ng/ml. The results clearly indicate that measurement of pepsinogen I may be useful in predicting gastric distress induced by amino-BP in osteoporosis.

Similar content being viewed by others
References
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
DeGroen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, Pryor-Tillotson S, Seleznick MJ, Pinkas H, Wang KK (1996) Esophagitis associated with the use of alendronate. New Engl J Med 335:1016–1021
Kane S, Borison NN, Brixner D (2004) Pharmacoeconomic evaluation of gastrointestinal tract events during treatment with risedronate or alendronate: a retrospective cohort study. Am J Manage Care 10:S216–S226
Rossini M, Bianchi G, Di Munno O, Giannini S, Minisola S, Sinigaglia L, Adami S (2006) Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int 17:914–921
Lichtenberger L, Romero JJ, Gibson GW, Blank MA (2000) Effect of bisphosphonates on surface hydrophobicity and phosphatidylcholine concentration of rodent gastric mucosa. Dig Dis Sci 45:1792–1801
Hills BA, Butler BD, Lichtenberger LM (1983) The gastric mucosal barrier; the hydrophobic lining to the lumen of the stomach. Am J Physiol 244:G561–G568
Lichtenberger LM (1995) The hydrophobic barrier properties of gastrointestinal mucus. Ann Rev Physiol 57:565–583
Lichternberger LM, Graziani LA, Dial EJ, Butler BD, Holls BA (1983) Role of surface-active phospholipids in gastric cytoprotection. Science 219:1327–1329
Anand BS, Romero JJ, Sanduja SK, Lichtenberger LM (1999) Phospholipid association reduces the gastric mucosal toxicity of aspirin in human subjects. Am J Gastroenterol 94:1818–1822
Derakhshan MH, El-Omar E, Oien K, Gillen D, Fyfe V, Crabtree JE, McColl KEL (2006) Gsatric histology, serological markers and age as predictors of gastric acid secretion in patients infected with Helicobactor pylori. J Clin Pathol 59:1293–1299
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Ohashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H (2001) Osteoporosis diagnostic criteria review committee: Japanese Society for Bone and mineral research. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 19:331–337
Bauer DC, Black D, Ensrud K, Thompson D, Hochberg M, Nevitt M, Musliner T, Freedholm D, for the Fracture Intervention Trial Research Group (2000) Upper Gastrointestinal tract safety profile of alendronate. Arch Intern Med 160:517–525
Taggart H, Bolognese MA, Lindsay R, Ettinger MP, Mulder H, Josse RG, Robert A, Zippel H, Adami S, Ernst TF, Stevens KP (2002) Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc 77:262–270
Greenspan S, Field-Munves E, Tonino R, Smith M, Petruschke R, Wang L, Yetes J, DePapp AE, Palmesano J (2002) Tolerability of once-weekly alendronate in patients with osteoporosis: a randomized, double-blind, placebo-controlled study. Mayo Clin Proc 77:1044–1052
Tremaine WJ, Khosla S (2002) Bisphosphonates and upper gastrointestinal tract: skeletal gain without visceral pain? Mayo Clin Proc 77:1029–1030
Samioff IM, Taggart RT (1987) Pepsinogens, pepsins, and peptic ulcer. Clin Invest Med 10:215–221
Kemppainen H, Raiha I, Sourander L (1997) Serum pepsinogen I and gastrin in peptic ulcer patients using nonsteroidal anti-inflammatory drugs. Hepatogastroenterology 44:1143–1146
Asaka M, Kimura T, Kudo M, Takeda H, Mitani S, Miyazaki T, Miki K, Graham DY (1992) Relationship of Helicobactor pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology 102:760–766
Kumagai T, Malaty HM, Graham DY, Hosogaya S, Misawa K, Furihata K, Ota H, Sei C, Tanaka E, Akamatsu T, Shimizu T, Kiyosawa K, Katsuyama T (1998) Acquisition versus loss of Helicobactor pylori infection in Japan: results from 8-year birth cohort study. J Infect Dis 178:717–721
Miki K, Urita Y (2007) Using serum pepsinogens wisely in a clinical practice. J Dig Dis 8:8–14
Roughead EE, McGeechan K, Sayer GP (2004) Bisphosphonate use and subsequent prescription of acid suppressants. Br J Clin Pharmacol 57:813–816
Yang YX, Lewis JD, Epstein S, Metz DC (2006) Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 296:2947–2953
Vestaagard P, Rejmark L, Mosekilde L (2006) Proton pump inhibitors, histamin H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 79:76–83
De Vries F, Cooper AL, Cockle SM, van Staa T-P, Cooper C (2009) Fracture risk in patients receiving acid-suppressant medication alone and in combination with bisphosphonates. Osteoporos Int 20:1989–1998
Gertz BJ, Holland SD, Kline WF, Matuszewski BK, Freeman A, Quan H, Lasseter KC, Mucklow JC, Porras AG (1995) Studies of the oral bioavailability of alendronate. Clin Pharmacol Ther 58:288–298
Lahner E, Annibale B, Fave GD (2009) Systemic review: impared drug absorption related to the co-administration of antisecretory therapy. Aliment Pharmacol Ther 29:1219–1229
Acknowledgements
The authors wish to thank Dr. Toshitsugu Sugimoto (Department of Internal Medicine, Shimane University) for his stimulating discussions. The authors would also like to thank Mrs. Shigeyuki Ishii (Teijin Pharmaceutical Co.) and Masao Inoue (Tokyo CRO Co.) for their kind provision of information.
Conflicts of interest
Masataka Shiraki received honorarium for the lectures from the pharmaceutical companies, which distributed amino bisphosphonate. No other conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiraki, M., Yamazaki, Y., Kuroda, T. et al. Serum level of pepsinogen significantly associated with gastric distress induced by amino-bisphosphonates. Osteoporos Int 22, 1717–1723 (2011). https://doi.org/10.1007/s00198-010-1374-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1374-3