Abstract
Summary
Bone loss and recovery in a receptor activator for nuclear factor κ B ligand (RANKL)-administered rat model was assessed. Microarchitecture, mineralization and strength deteriorated faster than ovariectomy (OVX). Recovery was dependent on the loss of trabecular elements and connections. Early recovery suggests a natural mechanism in rats to overcome excess RANKL, and may have implications for long-term bone loss.
Purpose
To compare a model for experimental osteoporosis that induces bone loss by injecting RANKL into rats to an OVX rat model, and measure subsequent recovery of bone architecture, mineralization, and mechanics after stopping injections.
Methods
Mature, healthy, female Wistar rats were divided into high-dose RANKL, low-dose RANKL, OVX, and vehicle control groups. The right proximal tibiae were micro-computed tomography (micro-CT) scanned in vivo every 2 weeks from week 0 to week 12 and every 4 weeks from week 12 to week 20. Bone architectural, mineralization, and mechanical changes were determined. Serum calcium, RANKL, anti-RANKL, and osteoprotegerin were measured at weeks 0, 6, and 20.
Results
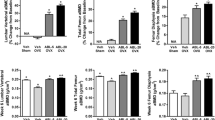
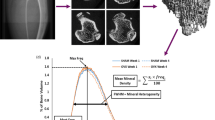
High-dose RANKL administration resulted in severe deterioration of the trabecular architecture (39% of baseline BV/TV), and modest decreases in tissue mineralization, bone mass, and stiffness. Bone loss occurred more rapidly than in the OVX and low-dose RANKL group, and recovery occurred prior to stopping RANKL injections. Full recovery of trabecular thickness, tissue mineralization, and cortical bone mass, partial recovery of trabecular bone volume (55% of baseline), structural model index, bone mass (69% of baseline), and stiffness (90% of baseline) but no improvement in connectivity density or trabecular number was observed.
Conclusion
RANKL administration resulted in rapid and dose-dependent bone loss. The recovery of trabecular bone volume and stiffness appeared to be dependent on the number of remaining trabecular elements and their interconnections. Uncontrolled recovery suggests that further investigation into the RANKL-injected rat as a model of bone loss is required.





Similar content being viewed by others
References
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137
Teitelbaum S (2000) Bone resorption by osteoclasts. Science 289:1504–1508
Marx J (2004) Coming to grips with bone loss. Science 305:1420–1422
(2001) Osteoporosis prevention, diagnosis, and therapy. Jama 285:785-795.
Kostenuik PJ (2005) Ostoprotegeren and RANKL regulate bone resorption, density, geometry and strength. Curr Opin in Pharmacol 5:618–625
Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci 96:3540–3545
Hofbauer LC, Schoppet M (2004) Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 292:490–495
Aufdemorte T, Boyan B, Fox C, Miller D (1993) Diagnostic tools and biological markers: animal models in the study of osteoporosis and oral bone loss. Journal of Bone and Mineral Research 8:S529–534
Dempster DW, Birchman R, Xu R, Lindsay R, Shen V (1995) Temporal changes in cancellous bone structure of rats immediately after ovariectomy. Bone 16:157–161
Campbell GM, Buie HR, Boyd SK (2008) Signs of irreversible architectural changes occur early in the development of experimental osteoporosis as assessed by in vivo micro-CT. Osteoporos Int 19:1409–1419
Boyd SK, Davison P, Mueller R, Gasser JA (2006) Monitoring individual morphological changes over time in ovariectomized rats by invivo micro computed tomography. Bone 39:854–862
Ominsky MS, Li X, Asuncion FJ, Barrero M, Warmington KS, Dwyer D, Stolina M, Geng Z, Grisanti M, Tan HL, Corbin T, McCabe J, Simonet WS, Ke HZ, Kostenuik PJ (2008) RANKL inhibition with osteoprotegerin increases bone strength by improving cortical and trabecular bone architecture in ovariectomized rats. J Bone Miner Res 23:672–682
Lloyd SA, Yuan YY, Kostenuik PJ, Ominsky MS, Lau AG, Morony S, Stolina M, Asuncion FJ, Bateman TA (2008) Soluble RANKL induces high bone turnover and decreases bone volume, density, and strength in mice. Calcif Tissue Int 82:361–372
Yuan YY, Kostenuik PJ, Ominsky MS, Morony S, Adamu S, Simionescu DT, Basalyga DM, Asuncion FJ, Bateman TA (2008) Skeletal deterioration induced by RANKL infusion: a model for high turnover bone disease. Osteoporos Int 19:625–635
Tomimori Y, Mori K, Koide M, Nakamichi Y, Ninomiya T, Udagawa N, Yasuda H (2009) Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J Bone Miner Res 24:1194–1205
Hofbauer LC, Heufelder AE (2001) Role of receptor activator of nuclear factor kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med 79:243–253
Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D (2004) The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev 15:457–475
Brouwers JE, van Rietbergen B, Huiskes R (2007) No effects of in vivo micro-CT radiation on structural parameters and bone marrow cells in proximal tibia of wistar rats detected after eight weekly scans. J Orthop Res 25:1325–1332
Klinck RJ, Campbell GM, Boyd SK (2007) Radiation effects on bone structure in mice and rats resulting from in vivo micro-computed tomography scanning. Med Eng Phys 30:888–895
Laib A, Kumer JL, Majumdar S, Lane NE (2001) The temporal changes of trabecular architecture in ovariectomized rats assessed by MicroCT. Osteoporos Int 12:936–941
Hildebrand T, Rüegsegger P (1997) A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc 185:67–75
Odgaard A (1997) Three-dimensional methods for quantification of cancellous bone architecture. Bone 20:315–328
Odgaard A, Gundersen HJ (1993) Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone 14:173–182
Hildebrand T, Ruegsegger P (1997) Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin 1:15–23
Boyd S, Moser S, Kuhn M, Klinck R, Krauze P, Müller R, Gasser J (2006) Evaluation of three-dimensional image registration methodologies for in vivo micro-computed tomography. Annals of Biomedical Engineering 34:1587–1599
van Rietbergen B, Weinans H, Huiskes R, Odgaard A (1995) A new method to determine trabecular bone elastic properties and loading using micromechanical finite element models. J Biomech 28:69–81
Su R, Campbell GM, Boyd SK (2006) Establishment of an architecture-specific experimental validation approach for finite element modeling of bone by rapid prototyping and high resolution computed tomography. Medical Engineering and Physics 29:480–490
Li X, Ominsky MS, Stolina M, Warmington KS, Geng Z, Niu QT, Asuncion FJ, Tan HL, Grisanti M, Dwyer D, Adamu S, Ke HZ, Simonet WS, Kostenuik PJ (2009) Increased RANK ligand in bone marrow of orchiectomized rats and prevention of their bone loss by the RANK ligand inhibitor osteoprotegerin. Bone 45:669–676
Acknowledgments
The authors wish to acknowledge the technical support of Mr. Jeffrey Louie for his assistance with the architectural analysis, Ms. Joan Miller for her assistance with the preparations of the injections, and Dr. Marina Stolina and Ms. Denise Dwyer for their assistance with the serum bioassays. Additionally, we would like to acknowledge the funding provided by the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Campbell, G.M., Ominsky, M.S. & Boyd, S.K. Bone quality is partially recovered after the discontinuation of RANKL administration in rats by increased bone mass on existing trabeculae: an in vivo micro-CT study. Osteoporos Int 22, 931–942 (2011). https://doi.org/10.1007/s00198-010-1283-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1283-5