Abstract
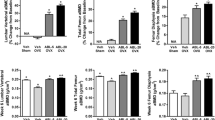
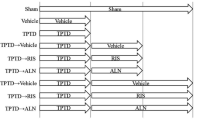
Anti-resorptive and anabolic treatments can be used sequentially to treat osteoporosis, but their effects on bone composition are incompletely understood. Osteocytes may influence bone tissue composition with sequential therapies because bisphosphonates diffuse into the canalicular network and anabolic treatments increase osteocyte lacunar size. Cortical bone composition of osteopenic, ovariectomized (OVX) rats was compared to that of Sham-operated rats and OVX rats given monotherapy or sequential regimens of single approved anti-osteoporosis medications. Adult female Sprague–Dawley rats were OVX (N = 37) or Sham–OVXd (N = 6). After 2 months, seven groups of OVX rats were given three consecutive 3-month periods of treatment with vehicle (V), h-PTH (1–34) (P), alendronate (A), or raloxifene (R), using the following orders: VVV, PVV, RRR, RPR, AAA, AVA, and APA. Compositional properties around osteocyte lacunae of the left tibial cortex were assessed from Raman spectra in perilacunar and non-perilacunar bone matrix regions. Sequential treatments involving parathyroid hormone (PTH) caused lower mean collagen maturity relative to monotherapies. Mean mineral:matrix ratio was 2.2% greater, mean collagen maturity was 1.4% greater, and mean carbonate:phosphate ratio was 2.2% lower in the perilacunar than in the non-perilacunar bone matrix region (all P < 0.05). These data demonstrate cortical bone tissue composition differences around osteocytes caused by sequential treatment with anti-osteoporosis medications. We speculate that the region-specific differences demonstrate the ability of osteocytes to alter bone tissue composition adjacent to lacunae.




Similar content being viewed by others
References
Gasser JA, Ingold P, Venturiere A et al (2008) Long-term protective effects of zoledronic acid on cancellous and cortical bone in the ovariectomized rat. J Bone Miner Res 23:544–551. https://doi.org/10.1359/jbmr.071207
Cosman F (2014) Anabolic and antiresorptive therapy for osteoporosis: combination and sequential approaches. Curr Osteoporos Rep 12:385–395. https://doi.org/10.1007/s11914-014-0237-9
Mosekilde L, Danielsen CC, Søgaard CH et al (1995) The anabolic effects of parathyroid hormone on cortical bone mass, dimensions and strength-assessed in a sexually mature, ovariectomized rat model. Bone 16:223–230. https://doi.org/10.1016/8756-3282(94)00033-V
Amugongo SK, Yao W, Jia J et al (2014) Effects of sequential osteoporosis treatments on trabecular bone in adult rats with low bone mass. Osteoporos Int 25:1735–1750. https://doi.org/10.1007/s00198-014-2678-5
Amugongo S, Yao W, Jia J et al (2014) Effect of sequential treatments with alendronate, parathyroid hormone (1–34) and raloxifene on cortical bone mass and strength in ovariectomized rats. Bone 67:257–268. https://doi.org/10.1097/OPX.0b013e3182540562
Boskey A, Mendelsohn R (2005) Infrared analysis of bone in health and disease. J Biomed Opt 10:31102. https://doi.org/10.1117/1.1922927
Acevedo C, Bale H, Gludovatz B et al (2015) Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone. Bone 81:352–363. https://doi.org/10.1016/j.bone.2015.08.002
Burr DB, Allen MR, Miller LM et al (2011) Bisphosphonates do not alter the rate of secondary mineralization. Bone 49:701–705. https://doi.org/10.1016/j.bone.2011.05.009
Bala Y, Depalle B, Farlay D et al (2012) Bone micromechanical properties are compromised during long-term alendronate therapy independently of mineralization. J Bone Miner Res 27:825–834. https://doi.org/10.1002/jbmr.1501
Burket JC, Brooks DJ, MacLeay JM et al (2013) Variations in nanomechanical properties and tissue composition within trabeculae from an ovine model of osteoporosis and treatment. Bone 52:326–336. https://doi.org/10.1016/j.bone.2012.10.018
Paschalis EP, Burr DB, Mendelsohn R et al (2003) Bone mineral and collagen quality in humeri of ovariectomized cynomolgus monkeys given rhPTH (1–34) for 18 months. J Bone Miner Res 18:769–775. https://doi.org/10.1359/jbmr.2003.18.4.769
Saito M, Marumo K, Kida Y et al (2011) Changes in the contents of enzymatic immature, mature, and non-enzymatic senescent cross-links of collagen after once-weekly treatment with h-PTH (1–34) for 18 months contribute to improvement of bone strength in OVX monkeys. Osteoporos Int 22:2373–2383. https://doi.org/10.1007/s00198-010-1454-4
Eswaran SK, Gupta A, Adams MF, Keaveny TM (2006) Cortical and trabecular load sharing in the human vertebral body. J Bone Miner Res 21:307–314. https://doi.org/10.1359/JBMR.051027
Stern A, Yao X, Wang Y et al (2018) Effect of osteoporosis treatment agents on the cortical bone osteocyte microenvironment in adult estrogen-deficient, osteopenic rats. Bone Rep 8:115–124. https://doi.org/10.1016/j.bonr.2018.02.005
Tazawa K, Hoshi K, Kawamoto S et al (2004) Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. J Bone Miner Metab 22:524–529. https://doi.org/10.1007/s00774-004-0519-x
Gardinier JD, Al-Omaishi S, Rostami N et al (2018) Examining the influence of PTH (1–34) on tissue strength and composition. Bone 117:130–137. https://doi.org/10.1016/j.bone.2018.09.019
Roelofs AJ, Stewart CA, Sun S et al (2012) Influence of bone affinity on the skeletal distribution of fluorescently labeled bisphosphonates in vivo. J Bone Miner Res 27:835–847. https://doi.org/10.1002/jbmr.1543
Wysolmerski JJ (2013) Osteocytes remove and replace perilacunar mineral during reproductive cycles. Bone 54(2):230–236. https://doi.org/10.1016/j.bone.2013.01.025
Qing H, Bonewald LF (2009) Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int J Oral Sci 1(2):59–65. https://doi.org/10.4248/ijos.09019
Tsourdi E, Jähn K, Rauner M, Busse B, Bonewald LF (2018) Physiological and pathological osteocytic osteolysis. J Musculoskelet Neuronal Interact 18(3):292–303
Nicolella DP, Moravits DE, Gale AM et al (2006) Osteocyte lacunae tissue strain in cortical bone. J Biomech 39:1735–1743. https://doi.org/10.1016/j.jbiomech.2005.04.032
Gardinier JD, Al-Omaishi S, Morris MD, Kohn DH (2016) PTH signaling mediates perilacunar remodeling during exercise. Matrix Biol 52–54:162–175. https://doi.org/10.1016/j.matbio.2016.02.010
Mandair GS, Morris MD (2015) Contributions of Raman spectroscopy to the understanding of bone strength. BoneKEy 4:1–8. https://doi.org/10.1038/bonekey.2014.115
Gamsjaeger S, Masic A, Roschger P et al (2010) Cortical bone composition and orientation as a function of animal and tissue age in mice by Raman spectroscopy. Bone 47:392–399. https://doi.org/10.1016/j.bone.2010.04.608
Taylor EA, Lloyd AA, Salazar-Lara C, Donnelly EL (2017) Raman and FT-IR mineral to matrix ratios correlate with physical chemical properties of model compounds and native bone tissue. Appl Spectrosc 71(10):2404–2410. https://doi.org/10.1177/0003702817709286
Gamsjaeger S, Robins SP, Tatakis DN et al (2017) Identification of pyridinoline trivalent collagen cross-links by Raman microspectroscopy. Calcif Tissue Int 100(6):565–574. https://doi.org/10.1007/s00223-016-0232-5
Beattie JR, Sophocleous A, Caraher MC et al (2019) Raman spectroscopy as a predictive tool for monitoring osteoporosis therapy in a rat model of postmenopausal osteoporosis. J Mater Sci Mater Med 30(2):25. https://doi.org/10.1007/s10856-019-6226-x
de Souza RA, Xavier M, da Silva FF et al (2012) Influence of creatine supplementation on bone quality in the OVX rat model: an FT-Raman spectroscopy study. Lasers Med Sci 27(2):487–495. https://doi.org/10.1007/s10103-011-0976-0
Boskey A, Mendelsohn R (2005) Infrared analysis of bone in health and disease. J Biomed Opt 10:031102. https://doi.org/10.1117/1.1922927
Saito M, Marumo K (2015) Effects of collagen crosslinking on bone material properties in health and disease. Calcif Tissue Int 97(3):242–261. https://doi.org/10.1007/s00223-015-9985-5
Bala Y, Seeman E (2015) Bone’s material constituents and their contribution to bone strength in health, disease, and treatment. Calcif Tissue Int 97(3):308–326. https://doi.org/10.1007/s00223-015-9971-y
Garnero P (2012) The contribution of collagen crosslinks to bone strength. BoneKey Rep 1:182. https://doi.org/10.1038/bonekey.2012.182
Gamsjaeger S, Buchinger B, Zoehrer R et al (2011) Effects of one year daily teriparatide treatment on trabecular bone material properties in postmenopausal osteoporotic women previously treated with alendronate or risedronate. Bone 49:1160–1165. https://doi.org/10.1016/j.bone.2011.08.015
Boskey AL, Spevak L, Weinstein RS (2009) Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos Int 20:793–800. https://doi.org/10.1007/s00198-008-0725-9
Durchschlag E, Paschalis EP, Zoehrer R et al (2006) Bone material properties in trabecular bone from human iliac crest biopsies after 3- and 5-year treatment with risedronate. J Bone Miner Res 21:1581–1590. https://doi.org/10.1359/jbmr.060701
Gamsjaeger S, Buchinger B, Zwettler E et al (2011) Bone material properties in actively bone-forming trabeculae in postmenopausal women with osteoporosis after three years of treatment with once-yearly zoledronic acid. J Bone Miner Res 26:12–18. https://doi.org/10.1002/jbmr.180
Gallant MA, Brown DM, Hammond M et al (2015) Bone cell-independent benefits of raloxifene on the skeleton: a novel mechanism for improving bone material properties. Bone 61:191–200. https://doi.org/10.1016/j.bone.2014.01.009.Bone
Roschger P, Lombardi A, Misof BM et al (2010) Mineralization density distribution of postmenopausal osteoporotic bone is restored to normal after long-term alendronate treatment: qBEI and sSAXS data from the fracture intervention trial long-term extension (FLEX). J Bone Miner Res 25:48–55. https://doi.org/10.1359/jbmr.090702
Ou-Yang H, Paschalis EP, Mayo WE et al (2001) Infrared microscopic imaging of bone: spatial distribution of CO3(2−). J Bone Miner Res 16:893–900. https://doi.org/10.1359/jbmr.2001.16.5.893
Donnelly E, Meredith DS, Nguyen JT et al (2012) Reduced cortical bone compositional heterogeneity with bisphosphonate treatment in postmenopausal women with intertrochanteric and subtrochanteric fractures. J Bone Miner Res 27:672–678. https://doi.org/10.1002/jbmr.560
Shah FA, Stoica A, Cardemil C, Palmquist A (2017) Multiscale characterisation of cortical bone composition, microstructure, and nanomechanical properties in experimentally-induced osteoporosis. J Biomed Mater Res 106(4):997–1007. https://doi.org/10.1002/jbm.a.36294
Miyagawa K, Kozai Y, Ito Y et al (2011) A novel underuse model shows that inactivity but not OVX determines the deteriorated material properties and geometry of cortical bone in the tibia of adult rats. J Bone Miner Metab 29(4):422–436. https://doi.org/10.1007/s00774-010-0241-9
Paolillo FR, Romano RA, de Matos L et al (2018) Short-term and long-term effects of osteoporosis on incisor teeth and femoral bones evaluated by Raman spectroscopy and energy dispersive X-ray analysis in OVX rats. J Bone Miner Metab 37(1):18–27. https://doi.org/10.1007/s00774-018-0903-6
Paschalis EP, Gamsjaeger S, Condon K, Klaushofer K, Burr D (2019) Estrogen depletion alters mineralization regulation mechanisms in an OVX monkey animal model. Bone 120:279–284. https://doi.org/10.1016/j.bone.2018.11.004
Paschalis EP, Gamsjaeger S, Hassler N, Klaushofer K, Burr D (2017) Ovarian hormone depletion affects cortical bone quality differently on different skeletal envelopes. Bone 95:55–64. https://doi.org/10.1016/j.bone.2016.10.029
Gourion-Arsiquaud S, Burket JC, Havill LM et al (2009) Spatial variation in osteonal bone properties relative to tissue and animal age. J Bone Miner Res 24:1271–1281. https://doi.org/10.1359/jbmr.090201
Qing H, Ardeshirpour L, Pajevic PD et al (2012) Remodeling in mice during lactation. J Bone Miner Res 27:1018–1029. https://doi.org/10.1002/jbmr.1567
Kaya S, Basta-Pljakic J, Seref-Ferlengez Z et al (2017) Lactation-induced changes in the volume of osteocyte lacunar-canalicular space alter mechanical properties in cortical bone tissue. J Bone Miner Res 32:676–680. https://doi.org/10.1002/jbmr.3090
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42:606–615. https://doi.org/10.1016/j.bone.2007.12.224
Rath Bonivtch A, Bonewald LF, Nicolella DP (2007) Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis. J Biomech 40:2199–2206. https://doi.org/10.1016/j.jbiomech.2006.10.040
Lane NE, Yao W, Balooch M et al (2006) Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res 21:466–476. https://doi.org/10.1359/JBMR.051103
Baylink DJ, Wergedal JE (1971) Bone formation by osteocytes. Am J Physiol 221:669–678
Villanueva AR, Ramser JR, Frost HM et al (1966) Tetracycline-based quantitative measurements of the tissue and cell dynamics in 10 cases of osteoporosis. Clin Orthop Relat Res 46:203–217
Frost HM (1969) Tetracycline-based histological analysis of bone remodeling. Calcif Tissue Res 3:211–239
Acknowledgements
We acknowledge Lynn Johnson, PhD, of the Cornell Statistical Consulting Unit for assistance with statistical analysis. This work was funded by National Institutes of Health Grants #’s R01 AR043052-07, 1 P50 AR03043, P50 AR060752NIH to NEL; The Endowment for Aging Research at UC Davis to NEL; and the Center for Musculoskeletal Health at UC Davis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Erik A. Taylor, Eve Donnelly, Xiaomei Yao, Mark L. Johnson, Sarah K. Amugongo, Donald B. Kimmel, and Nancy E. Lane declare that they have no conflict of interest or disclosures.
Human and Animal Rights and Informed Consent
We present no data from groups of human patients or individual human participants in this study. All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed during studies involving animals were in accordance with the ethical standards of practice at the University of California, Davis. This study only reports data from animal experiments for which the statement on animal welfare is included.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Taylor, E.A., Donnelly, E., Yao, X. et al. Sequential Treatment of Estrogen Deficient, Osteopenic Rats with Alendronate, Parathyroid Hormone (1–34), or Raloxifene Alters Cortical Bone Mineral and Matrix Composition. Calcif Tissue Int 106, 303–314 (2020). https://doi.org/10.1007/s00223-019-00634-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00634-w