Abstract
Summary
The study results indicate that women with osteoporosis initiated on gastro-resistant risedronate have a lower risk of fracture than those initiated on immediate release risedronate or alendronate. A large proportion of women discontinued all oral bisphosphonate therapies within 1 year of treatment start.
Purpose
Using a US claims database (2009–2019), we compared risk of fractures between women with osteoporosis initiated on gastro-resistant (GR) risedronate and those initiated on (a) immediate release (IR) risedronate or (b) immediate release alendronate.
Methods
Women aged ≥ 60 years with osteoporosis who had ≥ 2 oral bisphosphonate prescription fills were followed for ≥ 1 year after the first observed bisphosphonates dispensing (index date). Fracture risk was compared between the GR risedronate and IR risedronate/alendronate cohorts using adjusted incidence rate ratios (aIRRs), both overall and in subgroups with high fracture risk due to older age or comorbidity/medications. Site-specific fractures were identified based on diagnosis codes recorded on medical claims using a claims-based algorithm. Persistence on bisphosphonate therapy was evaluated for all groups.
Results
aIRRs generally indicated lower fracture risk for GR risedronate than IR risedronate and alendronate. When comparing GR risedronate to IR risedronate, statistically significant aIRRs (p < 0.05) were observed for pelvic fractures in the full cohorts (aIRRs = 0.37), for any fracture and pelvic fractures among women aged ≥ 65 years (aIRRs = 0.63 and 0.41), for any fracture and pelvic fractures among women aged ≥ 70 years (aIRRs = 0.69 and 0.24), and for pelvic fracture among high-risk women due to comorbidity/medications (aIRR = 0.34). When comparing GR risedronate to alendronate, statistically significant aIRRs were observed for pelvic fractures in the full cohorts (aIRR = 0.54), for any fracture and wrist/arm fractures among women aged ≥ 65 years (aIRRs = 0.73 and 0.63), and for any fracture, pelvic, and wrist/arm fractures among women aged ≥ 70 years (aIRRs = 0.72, 0.36, and 0.58). In all cohorts, ~ 40% completely discontinued oral bisphosphonates within 1 year.
Conclusions
Discontinuation rates of oral bisphosphonate therapy were high. However, women initiated on GR risedronate had a significantly lower risk of fracture for several skeletal sites than women initiated on IR risedronate/alendronate, particularly those aged ≥ 70 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis, a widespread bone disease associated with high risk of fractures and impaired quality of life [1], affects approximately one in four women and one in ten men worldwide across all ages, with higher prevalence in older patients and variations across countries [1, 2]. Osteoporosis is a clinical condition that is asymptomatic until it is complicated by low-trauma fractures, commonly of the wrist/arm, spine, hip, and/or pelvis [3, 4]. Osteoporotic fractures often require inpatient care and are associated with financial burdens for patients and their families, function loss, chronic pain, disability, and mortality [5, 6]. Among women aged ≥ 50 years in the USA, the age-adjusted prevalence of osteoporosis defined by dual-energy X-ray absorptiometry at either the femoral neck and/or lumbar spine has increased over time, from 14.0% in 2007–2008 to 19.6% in 2017–2018; the prevalence was also higher in women aged ≥ 65 years than those aged 50 to 64 years (27.1% vs. 13.1%, respectively, in 2017–2018 [1]).
For patients with osteoporosis, pharmacological therapy aims to reduce the risk of fractures [3, 7]. Oral bisphosphonates, the mainstay therapy for the prevention of osteoporotic fracture in the majority of postmenopausal women and men reduce the risk of fractures through multiple mechanisms of action including bone loss slowing and bone density improvement [8,9,10]. However, oral bisphosphonates have low (< 1–2%) oral bioavailability due to their poor lipophilicity, which leads to poor absorption in the gastrointestinal tract; furthermore, bioavailability may be reduced if the treatment dosing/administration instructions are not strictly followed [8]. Given patients must take oral bisphosphonates on an empty stomach followed by fasting and maintaining an upright position for 30 to 60 min, the administration of treatment is often perceived as inconvenient, which may explain why many patients do not comply with the dosing instructions [11] and/or discontinue treatment early [6, 12,13,14]. Indeed, a 2019 systematic review showed that approximately one-third to one-half of the post-menopausal women with osteoporosis were non-adherent to bisphosphonate therapy, and between 28 and 74% discontinued treatment within 1 year of treatment start [12]. Low adherence, including low compliance and low persistence, has been a concern for treating physicians as the reduction of osteoporosis fracture risk and fracture-related hospitalization rates depends on it [4, 8, 15].
Gastric-resistant (GR) risedronate was developed with the goal of providing a more convenient administration without impacting the overall bioavailability and efficacy of the treatment [8, 9]. GR risedronate combines the convenient once weekly with a “no fasting” dosing regimen, an enteric-coating that allows it to bypass the stomach to be absorbed in the small intestine, and an ethylenediaminetetraacetic acid (EDTA)-rich formulation that supports absorption by reducing divalent cation chelation that may interfere with its absorption [8]. However, while GR risedronate has been in clinical use for > 10 years, there are very few head-to-head comparisons of GR risedronate versus other oral bisphosphonates. Notable studies include a 2012–2013 non-inferiority randomized trial that compared the GR and IR formulations of risedronate [9, 11] and a 2021 real-world study that compared fracture risk between women initiated on risedronate GR and women initiated on other oral bisphosphonates [6], but both studies had limitations. The former was only powered to examine non-inferiority for the bone mineral density primary end point and did not have sufficient power to detect differences in fracture rates [9, 11]. The latter found that women initiated on risedronate GR had a lower incidence of any osteoporotic fractures and spine fractures than those initiated on other oral bisphosphonates (incidence rate ratio [IRR], 95% confidence interval [CI]: 0.83, 0.70–0.97 and 0.71, 0.54–0.95, respectively) and those initiated on alendronate (0.81, 0.66–0.98 and 0.69, 0.49–0.97) [6], but it remained unclear whether the observed differences between risedronate GR and other oral bisphosphonates/alendronate were due to the use of a different agent, the use of GR formulation, or both.
For the current study, we hypothesized that the increased bioavailability of GR risedronate would translate into lower risk of osteoporotic fracture (i.e., fracture of wrist/arm, spine, hip, and/or pelvis) for women initiated on this treatment compared to women initiated on oral bisphosphonates with IR formulations. Accordingly, our primary objective was to compare in a real-world setting the risk of fractures between women with osteoporosis initiated on GR risedronate and those initiated on (a) IR risedronate (a comparison in which the difference between cohorts is exclusively driven by the GR vs. IR formulation) and (b) alendronate (a comparison in which the difference between cohorts may be driven by either/both the GR vs IR formulation or/and the use of a different agent. Alendronate was selected as comparator because prior studies [6] suggested alendronate is the most commonly used oral bisphosphonate in the USA). In addition, we also hypothesized that the more convenient dosing schedule of GR risedronate, which does not require fasting, would translate into lower discontinuation rates. Accordingly, a secondary objective of the current study was to compare persistence on bisphosphonate therapy between GR risedronate and IR risedronate /alendronate.
Methods
Data source
This population-based retrospective study used claims data from the IBM® MarketScan® Commercial and Medicare Supplemental DatabasesFootnote 1 (Q1 2009 to Q3 2019). This large database includes de-identified patient-level claims from pharmacy and medical health services of approximately 130 million employees, dependents, and retirees in the USA who have healthcare coverage through employer-based commercial and Medicare supplemental health insurance plans. The data are compliant with the Health Insurance Portability and Accountability Act (HIPAA).
Study design
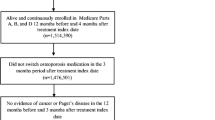
This is a retrospective cohort observational study. For each woman who satisfied the study inclusion criteria (see next paragraph; Fig. 1), the index date corresponded to the first dispensing for GR risedronate, IR risedronate, or alendronate (index treatment/study cohorts). By design, women who had a prescription fill for any other oral bisphosphonate before the index date were excluded from the sample. Unless otherwise specified, patient baseline characteristics were measured in the 6 months preceding the index date (baseline period, inclusive of index date), while fracture rate outcomes were assessed from the index date to the earliest of data cut-off date, end of insurance eligibility, or initiation of an oral bisphosphonate other than the index treatment (observation period). By design, the observation period was ≥ 1 year for all women. Persistence to therapy with any oral bisphosphonates was assessed from index date until the earliest of data cut-off date or end of insurance eligibility. A study design schematic is presented in Online Resource 1.
Selection of study sample and cohorts
Women eligible for inclusion in the study sample were aged ≥ 60 years at the index date, had ≥ 6 months of continuous healthcare plan enrollment prior to the index date, had ≥ 1 indicator of osteoporosis prior to or around the index date (i.e., ICD-9/10 diagnosis code for either osteoporosis or for osteoporotic fracture), had ≥ 1 year of continuous healthcare plan enrollment after the index date without initiation of a new oral bisphosphonate in the first year post-index, had no other bisphosphonate therapy indications (i.e., Paget’s disease, malignant neoplasms) during the baseline period or on the index date, and were initiated on one of the index treatments on the index date.
In addition, a criterion of ≥ 2 prescription fills for the index treatment with a maximum of 30-day gap between the last day supply of the prescription fill on the index date and the date of the next prescription fill was used as a surrogate for adherence to therapy (the 30-day-gap duration corresponded to the most commonly observed value in the data for the days’ supply associated with the treatments of interest). Figure 1 presents the study sample flowchart and additional details on the sample inclusion criteria. Online Resource 2 presents the diagnosis codes used in the sample selection.
All women initiated on GR risedronate on the index date were included in the GR risedronate cohort, while the IR risedronate and alendronate comparator cohorts were randomly selected from eligible women so that the index year distribution in these cohorts matched the index year distribution in the GR risedronate cohort. To maximize the number of women included in the comparator cohorts, a 1:1 selection ratio was used for IR risedronate, and a 1:13 selection ratio was used for alendronate (Fig. 1).
Definition of outcomes and statistical analyses
Osteoporotic fracture rates and bisphosphonate persistence outcomes were compared between the GR risedronate and the IR risedronate/alendronate cohorts both overall and in the following three subgroups of women considered to have high risk of fracture at the index date: (a) women aged ≥ 65 years [16], (b) women aged ≥ 70 years [16], and (c) women with other fracture risk factors (i.e., heart failure, chronic pulmonary disease, dementia, depression, diabetes, Parkinson’s disease comorbidities; prior osteoporotic fracture; or treatment with proton-pump inhibitors, sedatives, systemic corticosteroids, or loop diuretics [3, 17, 18], identified based on ≥ 1 relevant diagnosis code or treatment dispensing in the baseline period).
Fracture rates
Fracture events were identified from medical claims with osteoporotic fracture diagnosis code in the observation period (listed in Online Resource 2). Fracture incidence rates were measured overall and at major skeletal sites (hip, pelvis, spine, wrist/arm). A sensitivity analysis that excluded diagnosis codes of cervical fractures from the spine fracture site was used to assess the possible misclassification of cervical fractures as osteoporosis-related.
A definitional algorithm was applied to distinguish in the claims data between new fracture events and follow-up care associated with a prior fracture [19]. Specifically, if a woman had multiple claims for fracture, all fracture claims occurring at the same skeletal site (hip, pelvis, spine, or wrist/arm) within 90 days of the first claim and those occurring at a distinct skeletal site within 30 days of the first claim were considered follow-up care/fracture aftercare. The first claim at the same skeletal site ≥ 90 days after the first claim or at a distinct skeletal site ≥ 30 days after the first claim indicated a new fracture event. Thus, when calculating incidence rates for any fractures, it is possible that two consecutive fractures at the same major skeletal site were counted as one fracture event if they occurred within 90 days of each other, while two consecutive fractures at different skeletal site were counted as one fracture event if they occurred within 30 days of each other. Of note, because the latter scenario is considered as one fracture involving multiple major skeletal sites, in analyses reporting incidence rates by skeletal site, one fracture involving multiple skeletal sites will be counted as a fracture event for each of the skeletal sites involved.
For each cohort, the incidence rate of fractures was calculated as the number of fracture events divided by the total women-years of observation to account for different lengths of observation across women. The fracture incidence rates were compared between women initiated on GR risedronate and the comparator cohorts using generalized linear models with log link and Poisson/negative binomial distribution, which yielded unadjusted IRRs (models without covariates) and aIRRs (models adjusted for potential confounders) and 95% CIs. The potential confounders available in the data and adjusted for in the regression models included age category, census region, insurance plan type, Medicare coverage, year of the index date, comorbidities during the baseline period, Charlson comorbidity index, the presence of ≥ 1 fracture at any site prior to the index date (anytime), the presence of ≥ 1 dispensing for a drug decreasing the risk of fracture during the baseline period (listed in Table 1), the presence of ≥ 1 dispensing for a drug increasing the risk of fracture during the baseline period (listed in Table 1), and the number of days of supply of the first index prescription (≤ 30 days, > 30 days).
Persistence on the bisphosphonate treatment
Treatment persistence was defined as the time from index date to the discontinuation of all oral bisphosphonate therapies, where bisphosphonate treatment discontinuation was considered to occur at the last day supply of an oral bisphosphonate before a gap of > 90 days without any oral bisphosphonate treatment. Women who did not discontinue oral bisphosphonates were censored on the data cut-off date, or the end of insurance eligibility, whichever occurred first. Treatment persistence was compared between the GR risedronate versus IR risedronate/alendronate cohorts using time-to-event analyses, which included Kaplan–Meier plots (for discontinuation rates at one and two years post-index date) and Cox proportional hazards regression models (for unadjusted and adjusted hazard ratios (HR). The adjusted models controlled for the same potential confounders as the Poisson/negative binomial regression models for the risk of fracture outcomes, as listed above, and account for censoring.
Results
Patient characteristics
Among the 59,593 women with osteoporosis initiated on oral bisphosphonates who satisfied the study inclusion criteria, the first oral bisphosphonate fill was GR risedronate in 1.8% (n = 1,080), IR risedronate in 10.7% (n = 6351), and alendronate in 71.9% (n = 42,824). All 1080 women with the prescription fill for GR risedronate on index date were included in the GR risedronate cohort. The 1:1 selection ratio for GR risedronate and IR risedronate resulted in 1080 women included in the IR risedronate cohort (17.0% of all selected women eligible women initiated on IR risedronate), while the 1:13 selection ratio for GR risedronate and alendronate resulted in 14,040 women in the alendronate cohort (32.8% of all selected women initiated on alendronate).
Patient characteristics are described in Table 1. The median ages were 66, 66, and 67 years for women in the GR risedronate, IR risedronate, and alendronate cohorts, respectively (p < 0.05 for GR risedronate vs. alendronate). Clinical characteristics that were statistically different between the GR risedronate cohort and IR risedronate and/or alendronate cohorts included history of fracture at any skeletal site any time before the index date (14.7% vs. 14.3% and 20.8%*) as well as hypertension (40.0% vs 39.8% and 43.8%*), diabetes (14.6% vs. 11.7%* and 14.2%), vitamin D deficiency (12.7% vs 11.3% and 9.0%*), rheumatoid arthritis (5.4% vs 6.4% and 4.0%*), primary or secondary hyperparathyroidism (1.3% vs 2.9%* and 1.4%), baseline use of drugs that decrease the risk of fracture (beta blockers, denosumab, and estrogens; pooled: 34.1% vs 29.5%* and 30.5%*), baseline use of drugs that increase the risk of fracture (proton pump inhibitors: 25.4% vs 21.3%* and 17.7%*; opioids: 25.3% vs 25.5% and 30.5%*; systemic corticosteroids: 27.2% vs 24.7% and 22.7%*; loop diuretics: 6.2% vs 4.4% and 8.0%*), and overall burden of disease (Charlson Comorbidity Index ≤ 2: 95.5% vs 95.1% and 93.4%*; *indicates p < 0.05 vs GR risedronate).
Fracture rates
Fracture rates were measured over a median observation time of 29, 32, and 32 months for the GR risedronate, IR risedronate, and alendronate cohorts, respectively. In all three cohorts, the unadjusted fracture rates per 1000 women-years were numerically higher for the subgroups aged ≥ 70 years than the subgroups aged ≥ 65 years, the subgroups with high fracture risk due to comorbidity/medications, and the full study cohorts (Table 2). For example, the unadjusted rates of any fracture were 67.4 per 1000 women-years for those aged ≥ 70 years treated with GR risedronate versus 58.3, 59.8, and 50.6 for those aged ≥ 65 years, those at high fracture risk due to comorbidity/medications, and the full cohort treated with GR risedronate, respectively (IR risedronate: 97.3 vs. 88.2, 79.6, 64.8, respectively; alendronate: 101.5 vs. 87.2, 80.3, 66.8, respectively; Table 2). Across the three cohorts, the unadjusted rates of fracture were numerically lowest for GR risedronate cohort, regardless of the skeletal site or subgroup (Table 2).
In analyses adjusted for potential confounders (Fig. 2; Online Resource 3), there was a numerically lower risk of fracture among women initiated on GR risedronate compared to IR risedronate/alendronate, and statistical significance (p < 0.05) was reached for several comparisons overall, by skeletal site and/or within high-risk subgroups. Compared with IR risedronate, GR risedronate was associated with significantly lower risk of any fracture among women aged ≥ 65 years (aIRR = 0.63, 95% CI 0.46–0.86) and ≥ 70 years (0.69, 0.50–0.96) and with significantly lower risk of pelvic fracture both overall (0.37, 0.17–0.81) and in all high-risk subgroups (age ≥ 65 years: 0.41, 0.18–0.93; age ≥ 70 years: 0.24, 0.08–0.68; high risk based on comorbidity/medication: 0.34, 0.15–0.78). Compared with alendronate, GR risedronate was associated with significantly lower risk of any fracture among women aged ≥ 65 years (0.73, 0.58–0.91) and ≥ 70 years (0.72, 0.56–0.92), with significantly lower risk of pelvic fracture both overall (0.54, 0.29–0.99) and in women aged ≥ 70 years (0.36, 0.15–0.84), and with significantly lower risk of wrist/arm fractures among women aged ≥ 65 years (0.63, 0.41–0.95) and ≥ 70 years (0.58, 0.36–0.94) (Fig. 2; Online Resource 3).
Most aIRR estimates remained significant after applying a Bonferroni adjustment. Specifically, for the GR risedronate versus IR risedronate comparison, the aIRR estimates that remained significant included any fracture for the subgroup aged ≥ 65 years and pelvic fracture for all women, the subgroups aged ≥ 70 years and at high risk based on comorbidity/medication; for the GR risedronate versus alendronate comparisons, the aIRR estimates that remained significant included any fracture for subgroups aged ≥ 65 and ≥ 70 years, and pelvic fracture for women aged ≥ 70 years (Fig. 2).
Fracture rates and aIRRs did not change in a sensitivity analysis excluding diagnosis codes for cervical fractures (< 2% of all fracture events), which may not have been osteoporotic.
Persistence on bisphosphonate therapy after the index treatment initiation
Prescriptions with dosages per label accounted for 86% of all prescription fills for alendronate (per label daily dosages: 10 mg/day [81%] and 5 mg/day [5%]), 95% prescription fills for IR risedronate (5 mg/day), and 94% of all prescription fills for GR risedronate (5 mg/day).
Across all cohorts within 1 and 2 years of treatment initiation, ~ 40% and ~ 60% completely discontinued oral bisphosphonate therapy, respectively (Fig. 3). Given women who failed to receive a second prescription fill of the index treatment within 30 days of the first prescription’s last day supply were excluded from the current study, these are underestimates of the oral bisphosphonate true discontinuation rates (38.7%, 33.3%, and 29.0% of eligible women initiated on GR risedronate, IR risedronate, and alendronate, respectively, failed to receive a second prescription fill for the index treatment and were thus excluded; Fig. 1).
Persistence was not significantly different between the GR risedronate and the IR risedronate and alendronate cohorts in either unadjusted analyses (discontinuation rates at 1 year: 44% vs. 40% and 41%; discontinuation rates at 2 years 62% vs. 60% and 59%, respectively; log-rank p ≥ 0.05 for all comparisons; Fig. 3) or adjusted analyses (aHR, 95% CI: 1.03, 0.93–1.14 for GR risedronate vs IR risedronate; and 1.06, 0.99–1.15 for GR risedronate vs alendronate).
Discussion
Results from this real-world study are supportive of the hypothesis that the increased bioavailability expected from the GR formulation of risedronate translates into lower risk of fracture for women initiated on this treatment compared to women initiated on oral bisphosphonates with IR formulations, including both IR risedronate and alendronate. The protective effect of GR risedronate appeared to be most manifest for the women with osteoporosis aged ≥ 70 years where significant effects (p < 0.05) were observed for both any fracture (aIRRs = 0.69 and 0.72 vs. IR risedronate and alendronate, respectively), pelvic fractures (aIRRs = 0.24 and 0.36), and wrist/arm fractures (aIRR = 0.58 vs. alendronate). Statistically significant protective effects of GR risedronate (p < 0.05) were also observed for women aged ≥ 65 years for any fracture (aIRRs = 0.63 and 0.73 vs. IR risedronate and alendronate), pelvic fractures (aIRR = 0.41 vs. IR risedronate), and wrist/arm factures (aIRR = 0.63 vs. alendronate). A protective effect of GR risedronate for pelvic fractures was also observed in the overall cohorts (aIRRs = 0.37 and 0.54 vs. IR risedronate and alendronate, respectively) and among women at high-osteoporotic risk due to comorbidities and/or medications (aIRR = 0.34 vs. IR risedronate). The study results are relevant to clinicians because the incidence and prevalence of osteoporotic fractures increases with age [16], and osteoporotic fractures, including pelvic fractures, are associated with significant morbidity and mortality [20].
A secondary hypothesis of the current study was that the more convenient dosing schedule of GR risedronate, which did not require fasting, would translate into higher persistence (i.e., lower discontinuation rates) for patients initiated on this treatment. However, this hypothesis was not supported by the current study. In fact, in all three cohorts, large proportions completely discontinued oral bisphosphonates within 1 and 2 years of treatment initiation (approximately 40% within 1 year and approximately 60% within 2 years). Importantly, this discontinuation pattern was observed among women who had at least one refill after the initial prescription fill, which presumably included women who had a stronger intent to receive osteoporosis preventive therapy. While compliance with the dosing and administration instructions could not be assessed using claims data, it is possible the more convenient dosing schedule of GR risedronate impacts treatment adherence through improved compliance without any persistence benefit. Given GR risedronate is absorbed whether taken before or after the morning meal, improved absorption may result in better outcomes even when patients are equally adherent. Finally, bisphosphonates accumulate in bone matrix and can persist in the matrix for many years [21], which may also contribute to the observed effect. Further studies are needed to assess whether compliance with treatment is a possible mediator for the reduced risk of fracture observed in the current study for GR risedronate.
Given GR risedronate and IR risedronate therapies are only different in the type of formulation used, the GR formulation is likely the main driver for the protective effect of GR risedronate when compared to IR risedronate. In contrast, when comparing GR risedronate with alendronate, the GR formulation, the use of a different agent, and the use of a different dosage could have impacted the results, complicating the interpretation. Based on prior studies that had mixed results when comparing IR risedronate and alendronate [22, 23], a differential impact of the risedronate versus alendronate agents cannot be ruled out. However, in the current study, we observed similar trends when comparing GR risedronate with IR risedronate and alendronate and did not find significant differences in the fracture risk between IR risedronate and alendronate (sensitivity analyses; data not shown). Thus, it is plausible that the increased bioavailability of GR risedronate and possibly simplified dosing instructions explain the observed protective effect of GR risedronate when compared with alendronate [8, 9]. The fact that the protective effect of GR risedronate was observed over a relatively short period of time (median observation time of 29–32 months across the three cohorts) and among patients with high rates of early treatment discontinuation further suggests the impact of GR risedronate on fracture risk manifests soon after treatment initiation.
Interestingly, in the current study, the protective effect of GR risedronate appeared to be stronger for pelvis than other skeletal sites. While the reasons for a differential effect for pelvic factures remain unknown and the number of incident pelvic fractures was low (3.4–6.9 per 1000 women-years; Table 2), this study finding is nevertheless relevant for clinical practice due to the heavy disease burden associated with pelvic fractures. Specifically, osteoporotic pelvic fractures are often seen in very elderly patients with combined bone de-mineralization etiologies including osteoporosis and vitamin D deficiency/insufficiency [24], a frail and vulnerable subgroup of patients. Patients with a pelvic fracture have significant risk for morbidity and mortality [20]. Given a rise in the incidence of pelvic fractures has been observed recently [25], it is important that clinicians have confidence in treatment options to prevent pelvic fractures, particularly among elderly.
In the current study, the more convenient dosing schedule of GR risedronate did not translate into lower discontinuation rates. One possible explanation for this unexpected finding is the higher out-of-pocket cost of GR risedronate compared to other oral bisphosphonate therapies in the USA [6], similar to other chronic conditions where higher out-of-pocket costs correlate with lower compliance and persistence [26,27,28,29]. Furthermore, given persistence barriers are multifactorial, it is possible other factors have a stronger impact on persistence. Indeed, results from a systematic review that assessed 60 persistence studies using real-world data from over 4 million patients treated with oral bisphosphonates showed persistence rates similar to those reported in the current study (range: 17.7 to 74.8% 1 year after treatment initiation and 12.9 to 72.0% 2 years after treatment initiation [13]). This and other studies showing either high discontinuation rates and/or a decline over time in the number of patients who are initiated on osteoporosis therapy raised concerns about the suboptimal levels of fracture prevention among patients with osteoporosis and the unmet treatment needs in these patients [30,31,32,33]. Factors, identified by these authors as contributors to both the low use of osteoporosis therapy and the low persistence with therapy among the users, include patients’ concerns with rare adverse events of oral bisphosphonates (e.g., atypical femoral fractures or osteonecrosis of the jaw), physicians treating osteoporosis as a low-priority condition, and limited access to and reimbursement of osteoporosis diagnostic investigations such as DXA [30, 31]. Hence, as pointed out in prior studies, efforts need to be made to educate physicians and patients, and to clarify the favorable risk–benefit ratio of oral bisphosphonates for patients, physicians, and third-party payers alike [31].
To our knowledge, this is the first head-to-head study that compared risk of fracture between women initiated on GR risedronate and specific oral bisphosphonates with IR formulation in a real-world setting. Data on this topic are also sparse in clinical trial settings. Indeed, the only report of fracture rates in GR risedronate versus other oral bisphosphonates comes from a randomized control trial that had bone mineral density as primary outcome [9]. In this study, the proportion of patients experiencing clinical vertebral and non-vertebral fractures (listed as adverse events) up to 2 years post-randomization was comparable between patients randomized to IR risedronate (0.3% and 4.9%, respectively; N = 307 women) and patients randomized to GR risedronate taken immediately after breakfast (0.0% and 4.2%; N = 307 women) [9]. However, given this was a non-inferiority trial powered for a different primary outcome [9], the study did not have sufficient power to detect significant differences in fracture rates. While head-to-head comparisons of GR risedronate versus IR risedronate or alendronate are lacking, our estimates are roughly aligned with estimates from studies that reported fracture rates for oral bisphosphonates (the unadjusted risks for any fracture were 50.6–66.8 fracture events per 1000 women-years in the current sample vs. 15–81 fracture events per 1000 women-years in other studies [6, 34,35,36,37,38]; unadjusted risks for site-specific fractures: 3.4–27.4 vs. 3–14 fracture events per 1000 women-years, respectively [34, 38]).
In the current study, patient characteristics were adjusted for in analyses to eliminate confounding when comparing the risk of fracture and persistence between the cohorts. However, it is interesting to note some differences between the study cohorts at baseline. When comparing patients initiated on GR risedronate with those initiated on IR formulations, patients initiated on GR risedronate were significantly more likely to have higher risk of fracture at baseline based on comorbidity and medications, higher baseline use of proton pump inhibitors, and slightly higher baseline use of drugs decreasing the risk of fracture than both patients initiated on IR risedronate and those initiated on alendronate. When focusing on the GR risedronate versus alendronate comparison specifically, patients initiated on GR risedronate were significantly more likely to have vitamin D deficiency and rheumatoid arthritis and to use systemic corticosteroids in the baseline period and were significantly less likely to have hypertension, to have pre-index fractures at any site, or to use opioids in the baseline period than those initiated on alendronate. These differences in baseline characteristics suggest physicians may channel certain patients for specific oral bisphosphonates based on their perceived baseline risk or other characteristics that go beyond the general instructions in the osteoporosis treatment guidelines [3, 7, 10]. Given physicians’ rationale for the treatment choice is not available in claims data, our focus was on identifying factors that may impact outcomes to control for confounding rather than identifying factors that influence treatment choice. Further studies are needed to investigate this topic and to determine whether any baseline covariates modify the effect of oral bisphosphonate therapy on the risk of fractures. Of particular interest, for the latter would be the role vitamin D when combined with specific types of oral bisphosphonates, as prior studies have shown low vitamin D levels reduce the efficacy of oral bisphosphonates in general [39].
An unexpected finding in the current sample was that between a quarter and a third of the patients treated with risedronate and alendronate used systemic corticosteroids in the baseline period. Nevertheless, given the systemic corticosteroid labels recommend frequent monitoring for osteoporosis, it is plausible that women treated with systemic corticosteroids will be screened more often, resulting in an overrepresentation of women treated with systemic corticosteroids in our sample. Furthermore, we used the same measurement across the three cohorts to minimize the impact of any measurement error when comparing the study cohorts. Finally, the average duration of oral corticosteroid use in the baseline period was relatively moderate (50.4 days; data not shown), and adjustment for corticosteroids did not have a major impact on the results.
The study findings should be interpreted in the light of its limitations:
First, given the retrospective nature of the data used, these association-level results could not establish causal inference.
Second, the current data only included patients with commercial health plans and Medicare Supplemental health plan resulting in a sample that may not be representative of the general osteoporosis population. This can be perceived by the relatively young age of the women in our sample.
Third, the total number of fractures may have been underestimated or overestimated in the current study as they were based on a claims-based algorithm that did not count multiple fractures that occurred contemporaneously at the same skeletal site (hip, spine, pelvis, and arm/wrist) and relied on the timing between diagnoses to distinguish between a visit for the initial fracture event and subsequent visits for follow-up care. This claims-based algorithm, described in the “Methods” section, was applied because diagnosis codes recorded on administrative claims are for billing purposes and thus include limited clinical information. Of note, results remained consistent in sensitivity analyses in which the 90-day window to define a fracture episode was replaced by shorter (30-day) and longer (180- and 365-day) windows (Online Resource 4).
Fourth, claims data do not provide any information on how medications are consumed. To ensure patients had exposure to the oral bisphosphonates of interest, we required that all patients had at least two consecutive prescription fills for their index treatment. Overall, 38.7%, 33.3%, and 29.0% of women in the GR risedronate, IR risedronate, and alendronate cohorts, respectively, did not meet this criterion. As a result, the discontinuation rates reported in the current study are likely underestimating the true discontinuation rates among patients initiated on oral bisphosphonates.
Fifth, in our outcome analyses, a large number of potential confounders were adjusted for, but residual confounding may have remained from factors that are not available in claims data or are inadequately measured. For example, we may not have captured all factors considered by physicians when assessing the patient baseline risk of fracture and making treatment decisions, such as body mass index or family history of osteoporotic fractures. Similarly, many patients use over the counter multivitamins or over the counter vitamin D supplements [40] that are not captured in the claims data, which may contribute to unobserved confounding.
Sixth, relatively few fracture events were observed, particularly for pelvic fracture, which may have limited our ability to adequately control for confounding for this comparison. However, consistent with the results presented in Table 1 showing baseline characteristics were generally similar between the treatment groups, we also found that the unadjusted IRR estimates were similar to the adjusted IRR estimates across all comparisons (Online Resource 3), suggesting confounding was limited in the current study.
Finally, treatments that negatively or positively impact bone density (e.g., estrogens) may have been continued past index date or newly initiated after the index date. However, this would only impact the results if the decision to continue or initiate these treatments past index date was influenced by the type of oral bisphosphonate used, which seems unlikely.
The current study also showed that discontinuation rates of oral bisphosphonates remain high in the USA even among women who refilled their initial prescription fill, suggesting unmet patient needs in preventing fractures among women with osteoporosis [30,31,32,33] have yet to be addressed.
Notwithstanding its limitations, the current study has generated evidence suggesting that the lower fracture rates associated with GR risedronate observed in prior studies [6] are at least partially explained by advantages of GR formulations of oral bisphosphonates relative to IR formulations. This finding is consistent with data that the GR formulation of risedronate has better absorption properties because it bypasses the stomach [8, 41]. The protective effect of GR risedronate appeared to be stronger among women aged ≥ 70 years and among other subgroups with higher baseline risk of fracture based on comorbidity/medications.
Data Availability
The data that support the findings of this study are available from Truven Health Analytics, but restrictions apply to the availability of these data, which were used under a license agreement for the current study and, accordingly, are not publicly available. Access to the IBM® MarketScan® Commercial Database and the Medicare Supplemental Database can be requested by contacting Truven Health Analytics.
Code availability
Codes can be provided upon request.
Notes
MarketScan is a registered trademark of IBM Corporation in the USA, other countries, or both.
References
Sarafrazi N, Wambogo EA, Shepherd JA (2021) Osteoporosis or low bone mass in older adults: United States, 2017–2018. NCHS data brief 1–8
Salari N, Ghasemi H, Mohammadi L, et al (2021) The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. Journal of Orthopaedic Surgery and Research 16:. https://doi.org/10.1186/s13018-021-02772-0
Cosman F, de Beur SJ, LeBoff MS et al (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381. https://doi.org/10.1007/s00198-014-2794-2
Jeremiah MP, Unwin BK, Greenawald MH, Casiano VE (2015) Diagnosis and management of osteoporosis. Am Fam Physician 92:261–268. https://doi.org/10.1093/innovait/ins123
Lorentzon M, Johansson H, Harvey NC et al (2022) Osteoporosis and fractures in women: the burden of disease. Climacteric 25:4–10. https://doi.org/10.1080/13697137.2021.1951206
Thomasius F, Palacios S, Alam A et al (2022) Fracture rates and economic outcomes in patients with osteoporosis prescribed risedronate gastro-resistant versus other oral bisphosphonates: a claims data analysis. Osteoporos Int 33:217–228. https://doi.org/10.1007/s00198-021-06108-w
Adler RA, El-Hajj Fuleihan G, Bauer DC et al (2016) Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 31:16–35. https://doi.org/10.1002/jbmr.2708
Pazianas M, Abrahamsen B, Ferrari S, Russell RGG (2013) Eliminating the need for fasting with oral administration of bisphosphonates. Ther Clin Risk Manag 9:395–402. https://doi.org/10.2147/TCRM.S52291
McClung MR, Balske A, Burgio DE et al (2013) Treatment of postmenopausal osteoporosis with delayed-release risedronate 35 mg weekly for 2 years. Osteoporos Int 24:301–310. https://doi.org/10.1007/s00198-012-2175-7
Kanis JA, Cooper C, Rizzoli R, Reginster JY (2019) Executive summary of the European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Calcif Tissue Int 104:235–238. https://doi.org/10.1007/s00223-018-00512-x
McClung MR, Miller PD, Brown JP et al (2012) Efficacy and safety of a novel delayed-release risedronate 35 mg once-a-week tablet. Osteoporos Int 23:267–276. https://doi.org/10.1007/s00198-011-1791-y
Fardellone P, Lello S, Cano A et al (2019) Real-world adherence and persistence with bisphosphonate therapy in postmenopausal women: a systematic review. Clin Ther 41:1576–1588. https://doi.org/10.1016/j.clinthera.2019.05.001
Fatoye F, Smith P, Gebrye T, Yeowell G (2019) Real-world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open 9:1–18. https://doi.org/10.1136/bmjopen-2018-027049
Ross S, Samuels E, Gairy K et al (2011) A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value in Health 14:571–581. https://doi.org/10.1016/j.jval.2010.11.010
Vytrisalova M, Touskova T, Ladova K et al (2015) Adherence to oral bisphosphonates: 30 more minutes in dosing instructions matter. Climacteric 18:608–616. https://doi.org/10.3109/13697137.2014.995164
Becker DJ, Kilgore ML, Morrisey MA (2010) The societal burden of osteoporosis. Curr Rheumatol Rep 12:186–191. https://doi.org/10.1007/s11926-010-0097-y
Mortensen SJ, Mohamadi A, Wright CL et al (2020) Medications as a risk factor for fragility hip fractures: a systematic review and meta-analysis. Calcif Tissue Int 107:1–9. https://doi.org/10.1007/s00223-020-00688-1
Lary CW, Rosen CJ, Kiel DP (2021) Osteoporosis and dementia: establishing a link. J Bone Miner Res 36:2103–2105. https://doi.org/10.1002/jbmr.4431
Balasubramanian A, Zhang J, Chen L et al (2019) Risk of subsequent fracture after prior fracture among older women. Osteoporos Int 30:79–92. https://doi.org/10.1007/s00198-018-4732-1
Andrich S, Haastert B, Neuhaus E et al (2017) Excess mortality after pelvic fractures among older people. J Bone Miner Res 32:1789–1801. https://doi.org/10.1002/jbmr.3116
Glowacki J (2005) (2005) Bisphosphonate and bone. Ortho J Harvard Med School 25:64–67
Watts NB, Worley K, Solis AMY et al (2004) Comparison of risedronate to alendronate and calcitonin for early reduction of nonvertebral fracture risk: results from a managed care Administrative Claims Database. J Manag Care Pharm 10:142–151
Lindsay R, Watts NB, Lange JL et al (2013) Effectiveness of risedronate and alendronate on nonvertebral fractures: an observational study through 2 years of therapy. Osteoporos Int 24:2345–2352
Gleich J, Kußmaul AC, Steiner E et al (2022) High prevalence of missed information related on bone health in orthogeriatric patients with fragility fractures of the pelvis—an institutional register-based analysis. Osteoporos Int 33:901–907. https://doi.org/10.1007/s00198-021-06246-1
Lundin N, Huttunen TT, Enocson A et al (2021) Epidemiology and mortality of pelvic and femur fractures—a nationwide register study of 417,840 fractures in Sweden across 16 years: diverging trends for potentially lethal fractures. Acta Orthop 92:323–328. https://doi.org/10.1080/17453674.2021.1878329
Baker-Goering MM, Roy K, Howard DH (2019) Relationship between adherence to antihypertensive medication regimen and outof- pocket costs among people aged 35 to 64 with employer-sponsored health insurance. Prev Chronic Dis 16:1–6. https://doi.org/10.5888/pcd16.180381
Heidari P, Cross W, Crawford K (2018) Do out-of-pocket costs affect medication adherence in adults with rheumatoid arthritis? A systematic review. Semin Arthritis Rheum 48:12–21
Pawaskar MD, Xu L, Tang Y et al (2018) Effect of medication copayment on adherence and discontinuation in medicare beneficiaries with type 2 diabetes: a retrospective administrative claims database analysis. Diabetes Therapy 9:1979–1993. https://doi.org/10.1007/s13300-018-0489-y
Seaman K, Sanfilippo F, Bulsara M, et al (2020) Predictors of ceasing or reducing statin medication following a large increase in the consumer copayment for medications: a retrospective observational study. Public Health Research and Practice 30:. https://doi.org/10.17061/phrp29121905
Lems WF, Raterman HG (2017) Critical issues and current challenges in osteoporosis and fracture prevention. An overview of unmet needs. Therapeutic Advances in Musculoskeletal Disease 9:299–316. https://doi.org/10.1177/https
Fuggle NR, Curtis B, Clynes M et al (2020) The treatment gap: the missed opportunities for osteoporosis therapy. Bone 144:1–19. https://doi.org/10.1016/J.BONE.2020.115833
Jha S, Wang Z, Laucis N, Bhattacharyya T (2015) Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 30:2179–2187. https://doi.org/10.1002/jbmr.2565
Khosla S, Shane E (2016) A crisis in the treatment of osteoporosis. J Bone Miner Res 31:1485–1487. https://doi.org/10.1002/jbmr.2888
Yusuf AA, Cummings SR, Watts NB, et al (2018) Real-world effectiveness of osteoporosis therapies for fracture reduction in post-menopausal women. Archives of Osteoporosis 13:.https://doi.org/10.1007/s11657-018-0439-3
Reynolds AW, Liu G, Kocis PT, et al (2018) Comparison of osteoporosis pharmacotherapy fracture rates: analysis of a marketScan® claims database cohort. International Journal of Endocrinology and Metabolism 16:.https://doi.org/10.5812/ijem.12104
Modi A, Tang J, Sen S, Díez-Pérez A (2015) Osteoporotic fracture rate among women with at least 1 year of adherence to osteoporosis treatment. Curr Med Res Opin 31:767–777. https://doi.org/10.1185/03007995.2015.1016606
Liu J, Guo H, Rai P et al (2018) Medication persistence and risk of fracture among female Medicare beneficiaries diagnosed with osteoporosis. Osteoporos Int 29:2409–2417. https://doi.org/10.1007/s00198-018-4630-6
Curtis JR, Saag KG, Arora T et al (2020) Duration of bisphosphonate drug holidays and associated fracture risk. Med Care 58:419–426. https://doi.org/10.1097/MLR.0000000000001294
Carmel AS, Shieh A, Bang H, Bockman RS (2012) The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥33 ng/ml. Osteoporos Int 23:2479–2487. https://doi.org/10.1007/s00198-011-1868-7
Brincat M, Gambin J, Brincat M, Calleja-Agius J (2015) The role of vitamin D in osteoporosis. Maturitas 80:329–332. https://doi.org/10.1016/j.maturitas.2014.12.018
Kleinermans D, Joyson | Andrew, Wray | Heather, (2022) An open-label randomized study of the relative absorption of gastro-resistant risedronate taken fasted or with food versus immediate-release risedronate. Pharmacol Res Perspect 10:e00957. https://doi.org/10.1002/PRP2.957
Acknowledgements
Writing/editorial assistance in the preparation of this article was provided by Ariane Faucher from STATLOG Inc.
Funding
This study was funded by Theramex.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception, design, and interpretation of the data. JH, RII, and FV performed the analyses. All the authors provided constructive feedback during manuscript development and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable. Data are de-identified and comply with the Health Insurance Portability and Accountability Act (HIPAA) of 1996. Accordingly, this study did not require approval from an institutional review board or collection of inform consent.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JAE had received research support and/or consultation fees from Amgen, MSD, Lilly, Theramex. BC has conducted occasional work (consultancies, advice, conferences, clinical studies, courses) for Alexion, Amgen, Aptissen, Expanscience, Ferring, Lilly, Kyowa-Kirin, MSD, Novartis, Theramex, UCB, and Viatris. MB is an employee of Theramex. RII and FV are employees of STATLOG, Inc., which have received research funding from Theramex for this study. JH is an employee of Héroux Consulting, Inc., which has received research funding from STATLOG for this study. FT has received fees for lectures and consultancy or investigator fees from Amgen, Gedeon Richter, Lilly, Hexal, Kyowa Kirin, Hologic, Novartis, Stada, Synexus, Theramex, and UCB.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Eisman, J.A., Cortet, B., Boolell, M. et al. Fracture risk in women with osteoporosis initiated on gastro-resistant risedronate versus immediate release risedronate or alendronate: a claims data analysis in the USA. Osteoporos Int 34, 977–991 (2023). https://doi.org/10.1007/s00198-022-06627-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06627-0