Abstract
Introduction
Although both spinal cord injury (SCI) and sciatic neurectomy (NX) can cause osteopaenia in young rats, the effects of these two injuries on cortical and cancellous bone may differ. The objective of this study was to compare the effects of SCI and NX on bone weight, bone material property, bone mass, bone geometry, trabecular microarchitecture, mechanical strength and bone turnover in young rats.
Materials and methods
Thirty six-week-old male Sprague-Dawley rats were randomised into three groups (10 per group): SCI, bilateral sciatic NX and untreated control (CON). All rats were killed on day 21. Bone mineral density (BMD) was studied using dual-energy X-ray absorptiometry (DXA). At death, the right proximal tibial metaphysis and the fourth lumbar vertebra were examined for bone structural geometric analysis by micro-computed tomography (CT) and then processed for histomorphometry to assess bone cell activity. Serum N-terminal telopeptide of type I collagen (NTX) and osteocalcin (OC) levels were analysed by enzyme-linked immunosorbent assay (ELISA). Biomechanical strength properties of the femur and humerus were measured by three-point bending, and the third lumbar vertebra and the proximal end of tibia were tested by compression.
Results
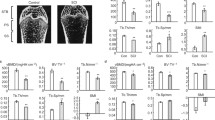
BMD in the sublesional areas of SCI rats was significantly lower than that of NX rats (proximal tibia, 0.176±0.018 g/cm2 vs. 0.224±0.015 g/cm2, P<0.001). Bone volume (BV/TV), trabecular number (Tb.N) and thickness (Tb.Th) in the tibial second spongiosa of SCI rats were significantly less than those in NX rats (BV/TV: 7.15±1.18% vs. 12.32±1.83%, P<0.001; Tb.N: 1.23±0.22 vs. 2.38±0.45, P<0.001; Tb.Th: 33.73±5.15 μm vs. 42.80±7.44 μm, P<0.01) and trabecular separation (Tb.Sp: 1,053.37±164.24 μm vs. 748.32±129.36 μm, P<0.01) was significantly greater than in NX rats. Furthermore, poorer trabecular connectivity was found in SCI rats than in NX rats (number of nodes, N.Nd/TV: 1.04±0.09 vs. 3.29±0.53; number of terminus, N.Tm/TV: 28.53±3.17 vs. 21.64±2.31, P<0.01). The bone formation rate of the tibial second spongiosa in SCI rats was significantly higher than in NX rats (2.06±0.13 vs. 1.53±0.09, P<0.001) and, also, the eroded surface in SCI rats was significantly higher than in NX rats (13.42±1.24 vs. 10.36±1.07, P<0.001). In addition, biomechanical tests showed that SCI rats had poorer biomechanical properties of the femur, proximal tibia and fourth lumbar vertebra than in NX rats. There were significantly higher levels of OC in SCI rats compared with NX rats (30.19±1.17 vs. 21.15±1.76, P<0.001). Also, serum NTX levels were significantly higher than in NX rats (51.60±2.61 vs. 33.85±1.93, P<0.001).
Conclusion
SCI caused more damage to bone mass, bone structure, biomechanical properties and bone metabolism than NX in young rats. This suggests that different mechanisms may underlie osteopaenia following SCI and NX.




Similar content being viewed by others
References
Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I (2000) Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. J Rehabil Res Dev 37(2):225–233
Takata S, Yasui N (2001) Disuse osteoporosis. J Med Invest 48(3–4):147–156
Uebelhart D, Demiaux-Domenech B, Roth M, Chantraine A (1995) Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia 33(11):669–673
Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF (2000) Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 27(2):305–309
Finsen V, Indredavik B, Fougner KJ (1992) Bone mineral and hormone status in paraplegics. Paraplegia 30(5):343–347
Biering-Sorensen F, Bohr HH, Schaadt OP (1990) Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest 20(3):330–335
Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM (1990) Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res 5(8):843–850
Maimoun L, Couret I, Micallef JP, Peruchon E, Mariano-Goulart D, Rossi M, Leroux JL, Ohanna F (2002) Use of bone biochemical markers with dual-energy x-ray absorptiometry for early determination of bone loss in persons with spinal cord injury. Metabolism 51(8):958–963
Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, McWhinney B, Hickman PE (1998) Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 83(2):415–422
Inoue M, Tanaka H, Moriwake T, Oka M, Sekiguchi C, Seino Y (2000) Altered biochemical markers of bone turnover in humans during 120 days of bed rest. Bone 26(3):281–286
Smith SM, Nillen JL, Leblanc A, Lipton A, Demers LM, Lane HW, Leach CS (1998) Collagen cross-link excretion during space flight and bed rest. J Clin Endocrinol Metab 83(10):3584–3591
Lueken SA, Arnaud SB, Taylor AK, Baylink DJ (1993) Changes in markers of bone formation and resorption in a bed rest model of weightlessness. J Bone Miner Res 8(12):1433–1438
Jones LM, Legge M, Goulding A (2002) Intensive exercise may preserve bone mass of the upper limbs in spinal cord injured males but does not retard demineralisation of the lower body. Spinal Cord 40(5):230–235
Jiang SD, Dai LY, Jiang LS (2006) Osteoporosis after spinal cord injury. Osteoporos Int 17(2):180–192
Iwamoto J, Takeda T, Ichimura S, Sato Y, Yeh JK (2003) Comparative effects of orchidectomy and sciatic neurectomy on cortical and cancellous bone in young growing rats. J Bone Miner Metab 21(4):211–216
Notoya K, Yoshida K, Tsukuda R, Taketomi S, Tsuda M (1996) Increase in femoral bone mass by ipriflavone alone and in combination with 1 alpha-hydroxyvitamin D3 in growing rats with skeletal unloading. Calcif Tissue Int 58(2):88–94
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2(6):595–610
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14(4):595–608
Jager I, Fratzl P (2000) Mineralized collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophys J 79(4):1737–1746
Glimcher MJ (1987) The nature of the mineral component of bone and the mechanism of calcification. Instr Course Lect 36:49–69
Bauman WA, Zhong YG, Schwartz E (1995) Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism 44(12):1612–1616
Bauman WA, Morrison NG, Spungen AM (2005) Vitamin D replacement therapy in persons with spinal cord injury. J Spinal Cord Med 28(3):203–207
Kikuchi TA, Skowsky WR, El-Toraei I, Swerdloff R (1976) The pituitary-gonadal axis in spinal cord injury. Fertil Steril 27(10):1142–1145
Naftchi NE, Viau AT, Sell GH, Lowman EW (1980) Pituitary-testicular axis dysfunction in spinal cord injury. Arch Phys Med Rehabil 61(9):402–405
Nance PW, Shears AH, Givner ML, Nance DM (1985) Gonadal regulation in men with flaccid paraplegia. Arch Phys Med Rehabil 66(11):757–759
Tsitouras PD, Zhong YG, Spungen AM, Bauman WA (1995) Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm Metab Res 27(6):287–292
Hjeltnes N, De Groot P, Birkeland KI, Falch JA, Iversen PO (2005) Tetraplegic subjects have hyperleptinaemia with marked circadian variation. Clin Endocrinol (Oxf) 62(2):223–227
Bugaresti JM, Tator CH, Silverberg JD, Szalai JP, Malkin DG, Malkin A, Tay SK (1992) Changes in thyroid hormones, thyroid stimulating hormone and cortisol in acute spinal cord injury. Paraplegia 30(6):401–409
Bauman WA, Spungen AM (1994) Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 43(6):749–756
Konttinen Y, Imai S, Suda A (1996) Neuropeptides and the puzzle of bone remodeling. State of the art. Acta Orthop Scand 67(6):632–639
Suzuki A, Palmer G, Bonjour JP, Caverzasio J (1998) Catecholamines stimulate the proliferation and alkaline phosphatase activity of MC3T3-E1 osteoblast-like cells. Bone 23(3):197–203
Chenu C, Serre CM, Raynal C, Burt-Pichat B, Delmas PD (1998) Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone 22(4):295–299
Itzstein C, Cheynel H, Burt-Pichat B, Merle B, Espinosa L, Delmas PD, Chenu C (2001) Molecular identification of NMDA glutamate receptors expressed in bone cells. J Cell Biochem 82(1):134–144
Peet NM, Grabowski PS, Laketic-Ljubojevic I, Skerry TM (1999) The glutamate receptor antagonist MK801 modulates bone resorption in vitro by a mechanism predominantly involving osteoclast differentiation. FASEB J 13(15):2179–2185
Sandhu HS, Herskovits MS, Singh IJ (1987) Effect of surgical sympathectomy on bone remodeling at rat incisor and molar root sockets. Anat Rec 219(1):32–38
Hill EL, Turner R, Elde R (1991) Effects of neonatal sympathectomy and capsaicin treatment on bone remodeling in rats. Neuroscience 44(3):747–755
Lundberg P, Lie A, Bjurholm A, Lehenkari PP, Horton MA, Lerner UH, Ransjo M (2000) Vasoactive intestinal peptide regulates osteoclast activity via specific binding sites on both osteoclasts and osteoblasts. Bone 27(6):803–810
Winding B, Wiltink A, Foged NT (1997) Pituitary adenylyl cyclase-activating polypeptides and vasoactive intestinal peptide inhibit bone resorption by isolated rabbit osteoclasts. Exp Physiol 82(5):871–886
Leslie WD, Nance PW (1993) Dissociated hip and spine demineralization: a specific finding in spinal cord injury. Arch Phys Med Rehabil 74(9):960–964
Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D (2001) Regional osteoporosis in women who have a complete spinal cord injury. J Bone Joint Surg Am 83-A(8):1195–1200
Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, Weinstein DA (1992) Osteoporosis after spinal cord injury. J Orthop Res 10(3):371–378
Szollar SM (1997) Osteoporosis in men with spinal cord injuries. West J Med 166(4):270
Biering-Sorensen F, Bohr H, Schaadt O (1988) Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia 26(5):293–301
Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S (1993) Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil 74(1):73–78
Muraki S, Yamamoto S, Ishibashi H, Horiuchi T, Hosoi T, Orimo H, Nakamura K (2004) Impact of degenerative spinal diseases on bone mineral density of the lumbar spine in elderly women. Osteoporos Int 15(9):724–728
Carter DR, Hayes WC (1977) The compressive behavior of bone as a two-phase porous structure. J Bone Joint Surg Am 59(7):954–962
Majumdar S, Kothari M, Augat P, Newitt DC, Link TM, Lin JC, Lang T, Lu Y, Genant HK (1998) High-resolution magnetic resonance imaging: three-dimensional trabecular bone architecture and biomechanical properties. Bone 22(5):445–454
Dempster DW (2000) The contribution of trabecular architecture to cancellous bone quality. J Bone Miner Res 15(1):20–23
Majumdar S, Link TM, Augat P, Lin JC, Newitt D, Lane NE, Genant HK (1999) Trabecular bone architecture in the distal radius using magnetic resonance imaging in subjects with fractures of the proximal femur. Magnetic Resonance Science Center and Osteoporosis and Arthritis Research Group. Osteoporos Int 10(3):231–239
Link TM, Majumdar S, Augat P, Lin JC, Newitt D, Lu Y, Lane NE, Genant HK (1998) In vivo high resolution MRI of the calcaneus: differences in trabecular structure in osteoporosis patients. J Bone Miner Res 13(7):1175–1182
Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM (1985) The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int 37(6):594–597
Nilsson A, Ohlsson C, Isaksson OG, Lindahl A, Isgaard J (1994) Hormonal regulation of longitudinal bone growth. Eur J Clin Nutr 48 Suppl 1:S150–S158; discussion S158–S160
Strange-Vognsen HH, Arnbjerg J, Hannibal J (1997) Immunocytochemical demonstration of pituitary adenylate cyclase activating polypeptide (PACAP) in the porcine epiphyseal cartilage canals. Neuropeptides 31(2):137–141
Agadir M, Sevastik B, Sevastik JA, Svensson L (1989) Effects of intercostal nerve resection on the longitudinal rib growth in the growing rabbit. J Orthop Res 7(5):690–695
Popiela H (1976) In vivo limb tissue development in the absence of nerves: a quantitative study. Exp Neurol 53(1):214–226
Dietz FR (1989) Effect of denervation on limb growth. J Orthop Res 7(2):292–303
Dysart PS, Harkness EM, Herbison GP (1989) Growth of the humerus after denervation. An experimental study in the rat. J Anat 167:147–159
Edoff K, Hellman J, Persliden J, Hildebrand C (1997) The developmental skeletal growth in the rat foot is reduced after denervation. Anat Embryol (Berl) 195(6):531–538
Yeni YN, Brown CU, Norman TL (1998) Influence of bone composition and apparent density on fracture toughness of the human femur and tibia. Bone 22(1):79–84
Pelker RR, Friedlaender GE, Markham TC, Panjabi MM, Moen CJ (1984) Effects of freezing and freeze-drying on the biomechanical properties of rat bone. J Orthop Res 1(4):405–411
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, SD., Jiang, LS. & Dai, LY. Spinal cord injury causes more damage to bone mass, bone structure, biomechanical properties and bone metabolism than sciatic neurectomy in young rats. Osteoporos Int 17, 1552–1561 (2006). https://doi.org/10.1007/s00198-006-0165-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0165-3