Abstract
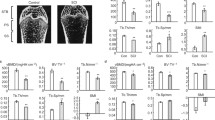
Sciatic nerve injury (SNI) can lead to significant bone loss in the lower extremities. However, the effects of SNI on the lumbar vertebrae are controversial. The present study aimed to evaluate the longitudinal effects of SNI on the lumbar vertebrae. Twenty-four 12-week-old male C57BL/6 mice (24.55±0.17 g) were randomly assigned to 3 groups (8 mice each) and underwent unilateral sciatic neurectomy (USN group), bilateral sciatic neurectomy (BSN group), or no surgery (CON group). The third (L3) and fourth (L4) lumbar vertebrae were scanned by in-vivo micro-computed tomography (ìCT) preoperatively and at 14 and 28 days postoperatively. Using μCT images, structural parameters and bone mineralization density distribution of the trabecular bone were analyzed among the 3 groups. In the BSN group, structural and material properties of L3 and L4 worsened after 14 days. Deterioration in the structural properties of L3 was observed at 28 days in the USN group, whereas no changes were observed in L4. These results implied that SNI can cause considerable deterioration in the microarchitecture of trabecular bone in the lumbar vertebrae. However, differences in the magnitude and rate of the deterioration and its onset period are observed between cases of unilateral and bilateral SNI.
Similar content being viewed by others
References
Turner, C., “Three Rules for Bone Adaptation to Mechanical Stimuli,” Bone, Vol. 23, No. 5, pp. 399–407, 1998.
Ko, C. Y., Jung, Y. J., Park, J. H., Seo, D., Han, P., et al., “Trabecular Bone Response to Mechanical Loading in Ovariectomized Sprague-Dawley Rats depends on Baseline Bone Quantity,” Journal of Biomechanics, Vol. 45, No. 11, pp. 2046–2049, 2012.
Ko, C. Y., Lee, T. W., Woo, D. G., Namgung, B. S., Kim, H. S., and Lee, B. Y., “Effect of Vibration on Lumbar Bone of OVX Rats Compared with Risedronate-Dosed Rat,” Proc. of IEEE International Conference on Biomedical and Pharmaceutical Engineering, pp. 201–205, 2006.
Lim, D., Ko, C. Y., Seo, D. H., Woo, D. G., Kim, J. M., Chun, K. J., and Kim, H. S., “LowIntensity Ultrasound Stimulation Prevents Osteoporotic Bone Loss in Young Adult Ovariectomized Mice,” Journal of Orthopaedic Research, Vol. 29, No. 1, pp. 116–125, 2011.
Woo, D. G., Ko, C. Y., Kim, H. S., Seo, J. B., and Lim, D., “Evaluation of the Potential Clinical Application of Low-Intensity Ultrasound Stimulation for Preventing Osteoporotic Bone Fracture,” Annals of Biomedical Engineering, Vol. 38, No. 7, pp. 2438–2446, 2010.
Kang, H., Ko, C. Y., Ryu, Y., Seo, D. H., Kim, H. S., and Jung, B., “Development of a Minimally Invasive Laser NEEDLE SYSTEM: Effects on Cortical Bone of Osteoporotic Mice,” Lasers in Medical Science, Vol. 27, No. 5, pp. 965–969, 2012.
Ko, C. Y., Kang, H., Ryu, Y., Jung, B., Kim, H., et al., “The Effects of Minimally Invasive Laser Needle System on Suppression of Trabecular Bone Loss Induced by Skeletal Unloading,” Lasers in Medical Science, Vol. 28, No. 6, pp. 1495–1502, 2013.
Ko, C. Y., Kang, H., Seo, D. H., Jung, B., Schreiber, J., and Kim, H. S., “Low-Level Laser Therapy using the Minimally Invasive Laser Needle System on Osteoporotic Bone in Ovariectomized Mice,” Medical Engineering & Physics, Vol. 35, No. 7, pp. 1015–1019, 2013.
Ito, M., Nishida, A., Nakamura, T., Uetani, M., and Hayashi, K., “Differences of Three-Dimensional Trabecular Microstructure in Osteopenic Rat Models Caused by Ovariectomy and Neurectomy,” Bone, Vol. 30, No. 4, pp. 594–598, 2002.
Ko, C. Y., Seo, D. H., and Kim, H. S., “Deterioration of Bone Quality in the Tibia and Fibula in Growing Mice during Skeletal Unloading: Gender-Related Differences,” Journal of Biomechanical Engineering, Vol. 133, No. 11, Paper No. 111003, 2011.
Morey-Holton, E. and Globus, R., “Hindlimb Unloading of Growing Rats: A Model for Predicting Skeletal Changes during Space Flight,” Bone, Vol. 22, No. 5, pp. 83S–88S, 1998.
Iwamoto, J., Takeda, T., Katsumata, T., Tanaka, T., Ichimura, S., and Toyama, Y., “Effect of Etidronate on Bone in Orchidectomized and Sciatic Neurectomized Adult Rats,” Bone, Vol. 30, No. 2, pp. 360–367, 2002.
Jiang, S. D., Jiang, L. S., and Dai, L. Y., “Spinal Cord Injury causes more Damage to Bone Mass, Bone Structure, Biomechanical Properties and Bone Metabolism than Sciatic Neurectomy in Young Rats,” Osteoporosis International, Vol. 17, No. 10, pp. 1552–1561, 2006.
Ko, C. Y., Jung, Y. J., Seo, D. H., Schreiber, J., Lim, D., and Kim, H. S., “Trabecular Bone Loss in Lumbar Vertebrae and Tibiae Following Sciatic Nerve Injury: Correlation between Baseline Bone Quantity (BV/TV) and the Magnitude and Rate Of Bone Loss,” Int. J. Precis. Eng. Manuf., Vol. 13, No. 9, pp. 1705–1708, 2012.
Ko, C. Y., Jung, Y. J., Seo, D. H., and Kim, H. S., “Bilateral Asymmetry in Microarchitecture of Trabecular Bone in Male c57bl/6 Mouse Tibia: Implication for Experimental Sample Size Estimations,” Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine, Vol. 227, No. 7, pp. 815–820, 2013.
Roschger, P., Paschalis, E. P., Fratzl, P., and Klaushofer, K., “Bone Mineralization Density Distribution in Health and Disease,” Bone, Vol. 42, No. 3, pp. 456–466, 2008.
Ruffoni, D., Fratzl, P., Roschger, P., Klaushofer, K., and Weinkamer, R., “The Bone Mineralization Density Distribution as a Fingerprint of the Mineralization Process,” Bone, Vol. 40, No. 5, pp. 1308–1319, 2007.
Sample, S. J., Collins, R. J., Wilson, A. P., Racette, M. A., Behan, M., et al., “Systemic Effects of Ulna Loading in Male Rats during Functional Adaptation,” Journal of Bone and Mineral Research, Vol. 25, No. 9, pp. 2016–2028, 2010.
Robling, A. G. and Turner, C. H., “Mechanotransduction in Bone: Genetic Effects on Mechanosensitivity in Mice,” Bone, Vol. 31, No. 5, pp. 562–569, 2002.
Robling, A. G., Warden, S. J., L Shultz, K., Beamer, W. G., and Turner, C. H., “Genetic Effects on Bone Mechanotransduction in Congenic Mice Harboring Bone Size and Strength Quantitative Trait Loci,” Journal of Bone and Mineral Research, Vol. 22, No. 7, pp. 984–991, 2007.
Klinck, J. and Boyd, S. K., “The Magnitude and Rate of Bone Loss in Ovariectomized Mice differs among Inbred Strains as Determined by Longitudinal in Vivo Micro-Computed Tomography,” Calcified Tissue International, Vol. 83, No. 1, pp. 70–79, 2008.
Padua, L., Caliandro, P., Bertolini, C., Calistri, A., Aprile, I., et al., “Post Traumatic Femoral Mononeuropathy,” Journal of Neurology, Vol. 253, No. 5, pp. 655–656, 2006.
Squire, M., Brazin, A., Keng, Y., and Judex, S., “Baseline Bone Morphometry and Cellular Activity Modulate the Degree of Bone Loss in the Appendicular Skeleton during Disuse,” Bone, Vol. 42, No. 2, pp. 341–349, 2008.
David, V., Laroche, N., Boudignon, B., LafageProust, M. H., Alexandre, C., et al., “Noninvasive in Vivo Monitoring of Bone Architecture Alterations in HindlimbUnloaded Female Rats using Novel ThreeDimensional Microcomputed Tomography,” Journal of Bone and Mineral Research, Vol. 18, No. 9, pp. 1622–1631, 2003.
Allen, M. R. and Bloomfield, S. A., “Hindlimb Unloading has a Greater Effect on Cortical Compared with Cancellous Bone in Mature Female Rats,” Journal of Applied Physiology, Vol. 94, No. 2, pp. 642–650, 2003.
David, V., Lafage-Proust, M. H., Laroche, N., Christian, A., Ruegsegger, P., and Vico, L., “Two-Week Longitudinal Survey of Bone Architecture Alteration in the Hindlimb-Unloaded Rat Model of Bone Loss: Sex Differences,” American Journal of Physiology-Endocrinology And Metabolism, Vol. 290, No. 3, pp. E440–E447, 2006.
Järvinen, T., Kannus, P., Pajamäki, I., Vuohelainen, T., Tuukkanen, J., et al., “Estrogen Deposits Extra Mineral into Bones of Female Rats in Puberty, but Simultaneously seems to Suppress the Responsiveness of Female Skeleton to Mechanical Loading,” Bone, Vol. 32, No. 6, pp. 642–651, 2003.
Turner, C. H., Takano, Y., and Owan, I., “Aging Changes Mechanical Loading Thresholds for Bone Formation in Rats,” Journal of Bone and Mineral Research, Vol. 10, No. 10, pp. 1544–1549, 1995.
Brodt, M. D., Ellis, C. B., and Silva, M. J., “Growing c57bl/6 Mice Increase whole Bone Mechanical Properties by Increasing Geometric and Material Properties,” Journal of Bone and Mineral Research, Vol. 14, No. 12, pp. 2159–2166, 1999.
Ahdjoudj, S., Lasmoles, F., Holy, X., Zerath, E., and Marie, P. J., “Transforming Growth Factor β2 Inhibits Adipocyte Differentiation induced by Skeletal Unloading in Rat Bone Marrow Stroma,” Journal of Bone and Mineral Research, Vol. 17, No. 4, pp. 668–677, 2002.
Dufour, C., Loly, X., and Marie, P. J., “Skeletal Unloading Induces Osteoblast Apoptosis and Targets Alpha5beta1-PI3K-Bcl-2 Signaling in Rat Bone,” Experimental Cell Research, Vol. 313, No. 2, pp. 394–403, 2007.
Basso, N., Jia, Y., Bellows, C. G., and Heersche, J. N., “The Effect of Reloading on Bone Volume, Osteoblast Number, and Osteoprogenitor Characteristics: Studies in Hind Limb Unloaded Rats,” Bone, Vol. 37, No. 3, pp. 370–378, 2005.
Aguirre, J. I., Plotkin, L. I., Stewart, S. A., Weinstein, R. S., Parfitt, A. M., et al., “Osteocyte Apoptosis is Induced by Weightlessness in Mice and Precedes Osteoclast Recruitment and Bone Loss,” Journal of Bone and Mineral Research, Vol. 21, No. 4, pp. 605–615, 2006.
Chenu, C., “Role of Innervation in the Control of Bone Remodeling,” Journal of Musculoskeletal and Neuronal Interactions, Vol. 4, No. 2, pp. 132, 2004.
Hill, E., Turner, R., and Elde, R., “Effects of Neonatal Sympathectomy and Capsaicin Treatment on Bone Remodeling in Rats,” Neuroscience, Vol. 44, No. 3, pp. 747–755, 1991.
Sherman, B. E. and Chole, R. A., “Sympathectomy, which Induces Membranous Bone Remodeling, has no Effect on Endochondral Long Bone Remodeling in Vivo,” Journal of Bone and Mineral Research, Vol. 15, No. 7, pp. 1354–1360, 2000.
Jiang, S. D., Jiang, L. S., and Dai, L. Y., “Mechanisms of Osteoporosis in Spinal Cord Injury,” Clinical Endocrinology, Vol. 65, No. 5, pp. 555–565, 2006.
Bugaresti, J., Tator, C., Silverberg, J., Szalai, J., Malkin, D., et al., “Changes in Thyroid Hormones, Thyroid Stimulating Hormone and Cortisol in Acute Spinal Cord Injury,” Spinal Cord, Vol. 30, No. 6, pp. 401–409, 1992.
Camacho-Hübner, C., Woods, K. A., Clark, A. J., and Savage, M. O., “Insulin-like Growth Factor (IGF)-I Gene Deletion,” Reviews in Endocrine & Metabolic Disorders, Vol. 3, No. 4, pp. 357–361, 2002.
Gamstedt, A., Järnerot, G., Kågedal, B., and Söderholm, B., “Corticosteroids and Thyroid Function,” Acta Medica Scandinavica, Vol. 205, No. 16, pp. 379–383, 1979.
Hjeltnes, N., De Groot, P., Birkeland, K. I., Falch, J. A., and Iversen, P. O., “Tetraplegic Subjects have Hyperleptinaemia with Marked Circadian Variation,” Clinical Endocrinology, Vol. 62, No. 2, pp. 223–227, 2005.
Couret, I., Micallef, J. P., Peruchon, E., Mariano-Goulart, D., Rossi, M., et al., “Use of Bone Biochemical Markers with Dual-Energy X-ray Absorptiometry for Early Determination of Bone Loss in Persons with Spinal Cord Injury,” Metabolism, Vol. 51, No. 8, pp. 958–963, 2002.
Pietschmann, P., Pils, P., Woloszczuk, W., Maerk, R., Lessan, D., and Stipicic, J., “Increased Serum Osteocalcin Levels in Patients with Paraplegia,” Spinal Cord, Vol. 30, No. 3, pp. 204–209, 1992.
Rosenquist, R. C., “Evaluation of 17-Ketosteroid, Estrogen and Gonadotrophin Excretion in Patients with Spinal Cord Injury,” The American Journal of Medicine, Vol. 8, No. 4, pp. 534–535, 1950.
Maimoun, L., Lumbroso, S., Paris, F., Couret, I., Peruchon, E., Rouays-Mabit, E., et al., “The Role of Androgens or Growth Factors in the Bone Resorption Process in Recent Spinal Cord Injured Patients: A Cross-Sectional Study,” Spinal Cord, Vol. 44, No. 12, pp. 791–797, 2006.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chang-Yong Ko and Young Jin Jung contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ko, CY., Jung, Y.J., Seo, D.H. et al. Deterioration of trabecular bone microarchitecture in the lumbar vertebrae in growing male mice following sciatic neurectomy. Int. J. Precis. Eng. Manuf. 15, 2605–2610 (2014). https://doi.org/10.1007/s12541-014-0633-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12541-014-0633-1