Abstract
Introduction and hypothesis
Macroplastique® (polydimethylsiloxane injection) is a minimally invasive urethral bulking agent with global clinical literature describing its use over 20 years. This study critically assessed the safety and effectiveness outcomes for adult women treated with Macroplastique for stress urinary incontinence (SUI) through a systematic review and meta-analysis.
Methods
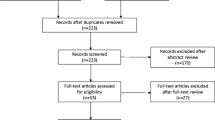
A systematic review of the scientific literature from 1990 to 2010 was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to quantitatively summarize the safety and effectiveness of Macroplastique for female SUI. A total of 958 patients from 23 cohorts were eligible for inclusion and were analyzed. Random-effects models were used to estimate the improvement and cure rates following treatment at three time periods: short-term (<6 months), mid-term (6–18 months), and long-term (>18 months). Expanded models assessed the effect of reinjection rate on successful treatment outcomes. Adverse event rates were aggregated and reported.
Results
Improvement rates were 75 % [95 % confidence interval (CI), 69–81] in the short-term, 73 % (95 % CI, 62–83) in the mid-term, and 64 % (95 % CI, 57–71) long-term. Cure/dry rates were 43 % (95 % CI, 33–54), 37 % (95 % CI, 28–46), and 36 % (95 % CI, 27–46) over the same respective follow-up periods. Higher study reinjection rates were associated with improved long-term SUI outcomes. No serious adverse events were reported.
Conclusions
This quantitative review supports Macroplastique as an effective, durable, and safe treatment option for female SUI. Meta-analytic evidence suggests that long-term therapeutic benefit is frequently maintained, with some patients requiring reinjection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) is a socially, emotionally, and physically devastating condition affecting millions of women worldwide. SUI is self-reported in up to 25 % of the adult female population in the United States [1]. Standard treatments include pelvic floor muscle exercise regimes, biofeedback, urethral slings, tension-free vaginal tapes (TVT), and urethral bulking agents (UBAs). Midurethral slings are the most frequently performed and effective surgical intervention for SUI. UBAs have been recommended by the American Urology Association for patients who do not wish to undergo a more invasive surgery, elderly patients, and patients at higher risk for anesthetic complications [2]. Guidance from the Society of Obstetricians and Gynaecologists of Canada Urogynaecology Committee, the American Congress of Obstetricians and Gynecologists, and the National Institute for Health and Clinical Excellence (NICE), among others, directs patients with significantly decreased urethral mobility and/or patients who have failed appropriate conservative therapy to consider periurethral bulking agents as one of several treatment options [3–5].
In 2011, glutaraldehyde-treated bovine collagen (Contigen®, CR Bard), the UBA with the longest history of use for female SUI, was withdrawn from worldwide availability, posing a pressing need for the urological community to evaluate alternative UBAs. New synthetic UBAs were developed; however, several—such as polytetrafluoroethylene, dextranomer/hyaluronic acid, and ethylene vinyl alcohol copolymer—were subsequently withdrawn or discontinued for safety or efficacy concerns. Currently, carbon-coated beads, calcium hydroxylapatite (CaHA), and polydimethylsiloxane are marketed to treat female SUI in the United States, whereas autologous cellular therapy, autologous fat, and autologous ear chondrocytes lack sufficient data on efficacy and safety and are considered investigational. Continence outcomes from randomized controlled trials of UBAs suggest that the efficacy of carbon-coated spheres, CaHA, and polydimethylsiloxane is similar to cross-linked collagen [6–8].
Polydimethylsiloxane (Macroplastique®, Uroplasty, Inc., Minnetonka, MN, USA) has a long history in the treatment of female SUI, primarily in Europe, where it has been used since 1991 and is the leading UBA outside the United States. Macroplastique is indicated to treat SUI primarily due to intrinsic sphincter deficiency (ISD). [Medicare, a federal program in the United States that provides for certain health care expenses for people ≥65, defines ISD in women with SUI as abdominal leak point pressures (ALPP) of ≤100 cm H2O without urethral hypermobility.] Macroplastique is nonallergenic, supplied in a ready to use form, and may be carried out in an office setting under local anesthesia. Macroplastique is comprised of highly textured silicone elastomer implants suspended in a polyvinylpyrrolidone (PVP) carrier matrix (Fig. 1). Macroplastique is implanted at several positions around the urethra, under direct vision with a cystoscope, approximately 1 cm distal to the bladder neck to reduce urine leakage from the bladder by bulking and coapting the urethral tissue left open by the weakened sphincter. The PVP carrier matrix is exchanged for tissue fluids containing host fibroblasts. The large, heavily textured elastomer implants are conducive to tissue in-growth, agglomerating and creating a bolus surrounded and infiltrated by host collagen. The fibrous encapsulation of the bolus anchors it within the space between the lamina propria and the urethral muscularis, preventing subsequent implant movement or migration. After implantation, the PVP carrier is resorbed by the reticuloendothelial system and excreted via glomerular filtration without being metabolized.
In 2003, a systematic review of Macroplastique concluded too few prospective, randomized studies had been conducted to reach definitive conclusions about its effectiveness [9]. Since then, a randomized controlled trial (RCT) comparing the effectiveness of Macroplastique to collagen was carried out [8], leading to marketing approval in the United States in 2006. Additionally, three other randomized studies comparing Macroplastique to pelvic floor muscle exercise therapy, pubovaginal sling surgery, and porcine dermal implant injection have been published [10–12]. These trials are complemented by several prospective studies of small cohorts and descriptive reports from clinical practice.
The goal of our study was to systematically review the scientific literature and meta-analyze Macroplastique treatment outcomes for treatment of adult female SUI over time. Additionally, we sought to investigate the effect of reinjection rates on improvement and cure rates and to aggregate reports of adverse events (AEs) to characterize treatment safety.
Methods
Study design and data sources
This systematic review and meta-analysis was conducted using the guidelines set forth in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [13]. We searched Ovid MEDLINE, PubMed, and the Cochrane Library databases from January 1990 to June 2010 and checked the references of systematic reviews for studies reporting Macroplastique treatment in women with SUI. Search terms were all pairwise combinations of the following terms: “Macroplastique,” “stress urinary incontinence,” and “silicone injection,” as well as “Macroplastique” alone.
Inclusion and exclusion criteria
Two statistical consultants (The Integra Group, Brooklyn Park, MN, USA) evaluated articles independently and in duplicate, and disagreements were resolved by consensus. Candidates for inclusion were peer-reviewed publications of RCTs and prospective, observational, or cohort studies reporting treatment outcomes and/or AEs related Macroplastique treatment for female SUI. Case studies, letter reports, reviews, and animal studies were excluded. To avoid bias, we also excluded statistics for which 25 % of the sample was lost to follow-up. In cases in which data from a particular study sample appeared in more than one publication, the article with the most complete follow-up data was used. We imposed no search restrictions based on language.
Data extraction and quality assessment
Data were extracted into a database by the second author and were double-checked independently by another statistical consultant for accuracy. The metric for assessing treatment efficacy varied between studies (e.g., Stamey incontinence grade, physician and patient ratings of improvement, pad weight), so we characterized treatment success according to the proportion of the study sample “cured” (i.e., defined as no symptoms of SUI, Stamey grade 0, or dryness) or “improved” (i.e., defined as cured or significant improvement in symptoms from baseline). In cases in which physician and patient assessments were reported, physician evaluations were used.
Follow-up periods from published studies varied from 1 month to >5years. Improvement and cure rates were classified into three time strata: short-term (<6 months), mid-term (6–18 months), and long-term (>18 months). The proportion of patients improved and cured was meta-analyzed for each time stratum. For retrospective studies in which improvement and cure rates were not collected at a prespecified time, we used the sample’s mean follow-up time to classify the time stratum. Where available, data on reinjection rates within studies were extracted to explore potential relationships with treatment success. Reported AEs were also extracted; however, clinical definitions varied too greatly between studies for meta-analysis. We report the median value and interquartile range (IQR) for AE rates across studies.
We evaluated methodological quality of all articles meeting inclusion criteria in several domains:
-
1.
description of the study population and selection method;
-
2.
description of potential confounders (e.g., urethral hypermobility and history of incontinence surgery);
-
3.
reporting of study location(s) and enrollment/treatment dates;
-
4.
description of treatment exposure (e.g., injection method, average volume injected, number of treatments, reinjection rates);
-
5.
appropriate statistical analysis;
-
6.
description of randomization, blinding, and Institutional Review Board approval, where applicable;
-
7.
description of loss to follow-up and the handling of missing data;
-
8.
reporting of AEs.
Statistical analysis
Significant heterogeneity between study outcomes was anticipated due to the small sample sizes of several studies and different designs. Therefore, we used random-effects (RE) linear regression models to estimate the proportions of the sample improved and cured at each of the three time strata. We applied an arcsine square-root transformation to stabilize variance estimates where necessary to ensure confidence limits were not estimated to be <0 % or >100 % [14]. Results from models using the arcsine-transformed data are reported back-transformed, so interpretation is identical for all models regardless of data transformation. Forest plots show study-specific and model estimates for each endpoint and indicate whether the arcsine transformation was applied. Expanded RE models were used to explore the effect of study reinjection rates on improvement and cure rates. Study heterogeneity, which describes the variation between study outcomes not due to chance variation, was measured using I2 (the percent of total variability in study outcomes attributable to heterogeneity) and was tested for significance using Cochran’s Q test. Funnel-plot asymmetry, an indicator of potential reporting bias, was assessed with Egger’s test. Statistical analyses were conducted with the metafor package [15] for R, version 2.14.1 (R Development Core Team, 2011).
Results
Study and sample characteristics
Sixty-five unique candidate articles were retrieved from all searches, and 23 patient cohorts from 24 published articles [8, 10–12, 16–35] met inclusion criteria and were included in the meta-analysis, for a total of 958 patients. Four patient cohorts were from randomized studies [8, 10–12], nine were prospective observational studies [16–23, 32], and ten were retrospective studies of clinical practice [24–30, 33–35]. We did not include 27 review papers, four case reports, nine studies that did not use Macroplastique, and one animal study. We excluded long-term data from three papers with insufficient follow-up [11, 31, 34]. All studies used a transurethral injection technique with either endoscopic injection or the Macroplastique Implantation System (MIS; Uroplasty BV, Geleen, The Netherlands), which is a nonendoscopic needle guide. Most studies reviewed defined ISD as maximum urethral pressure (MUP) or maximum closing urethral pressure (MUCP) ≤20 cm H2O and/or Valsalva’s leak-point pressure (VLPP/ALPP) <60 cm H2O.
Summaries of each study, including reported AEs and assessments for risk of bias according to the previously defined criteria, are presented in Table 1. Median age of study samples was 59 years (IQR, 54–65). The median proportion of the study samples that had undergone at least one previous surgery for urinary incontinence was 42 % (IQR, 30–70).
Most deviations from the expectation of quality were minor; however, two studies did not provide an adequate definition of improvement and cure. Both were included in meta-analyzed proportions despite contributing outlying data points [23, 25].
Meta-analysis of success and cure rates
Data from 12 studies contributed to the aggregate short-term improvement rate of 75 % [95 % confidence interval (CI), 69–81; (Fig. 2a)]. Thirteen studies contributed to the short-term cure rate of 43 % (95 % CI, 33–54; Fig. 2b). Significant heterogeneity was observed in both short-term improvement (I2 = 67 %, P = 0.05) and cure (I2 = 83 %, P < 0.001) rates but not publication bias (both P > 0.10).
Data from ten studies contributed to the mid-term improvement rate of 73 % (95 % CI, 62–83; Fig. 3a) and 11 to the cure rate of 37 % (95 % CI, 28–46; Fig. 3b). Significant heterogeneity was detected in mid-term improvement (I2 = 83 %, P < 0.001) and cure (I2 = 72 %, P < 0.001) rates but not publication bias (both P > 0.10).
Data from ten studies contributed to the aggregate long-term improvement rate of 64 % (95 % CI, 57–71; Fig. 4a) and 11 to the long-term cure rate of 36 % (95 % CI, 27–46; Fig. 4b). Significant heterogeneity was observed for long-term improvement (I2 = 56 %, P < 0.001) and cure (I2 = 79 %, P < 0.001) rates. Significant funnel plot asymmetry was detected in the long-term improvement rate, where studies with larger sampling errors (i.e., smaller sample sizes) reported lower improvement rates (P = 0.04). Conversely, there was a significant trend of studies with larger sampling errors reporting higher cure rates (P = 0.003).
Effect of reinjection on improvement and cure rates
Seventeen studies reported reinjection rates for patients who had not been cured after the first injection and thus offered reinjections to improve the outcome. Across these 17 studies, the median reinjection rate was 30 % (IQR 14–37). Expanded RE models found that higher reinjection rates were associated with higher improvement rates in long-term but not short-term or mid-term follow-up (both P > 0.10). On average, in long-term follow-up, 63 % (95 % CI, 56–70) of patients in a cohort with the typical reinjection rate (30 %) had SUI symptom improvement; each 10 % increase in the proportion of the sample undergoing repeat injections was associated with an additional 4 % (95 % CI, 1–7) of the sample having improved in the long term. Study reinjection rates were not significantly associated with study cure rates at any time point (all P > 0.3).
Adverse events
The median rates for AEs were 7 % (IQR, 5–15) for temporary urinary retention, 7 % (IQR, 4–27) for urge incontinence, 3 % (IQR, 0–8) for urinary tract infections, 50 % (IQR, 11–79) for temporary dysuria, and 45 % (IQR, 8–64) for transient hematuria. There were no reports of extrusion, migration, immune reaction, embolic phenomena, vascular occlusion, or other serious AEs.
Discussion
This systematic review and meta-analysis of 20 years of global experience in clinical research and practice indicates that Macroplastique is a safe and effective urethral bulking agent for treating women with SUI primarily due to ISD. Pooled results suggest successful outcomes in the short term for 75 % and cures for 43 % of patients. Furthermore, our results suggest that those with initial success frequently sustained therapeutic benefit past 18 months. On average, at the sample level, 85 % of patients sustained their improvement and 84 % sustained their cure, attesting to the durability of Macroplastique therapy. Ghoniem and colleagues followed for 2 years initially successful 12-month patients from the 122 patients treated in the US Investigation Device Exemption (IDE) RCT [31]. Though an insufficient number of patients were followed to include in the our meta-analysis, subcohort results were consistent with the meta-analyzed results reporting that of initially successful patients presenting at 2 years, 56 of 67 patients (84 %) maintained improvement at 2 years and 45 of 67 (67 %) maintained cure.
The long-term reinjection rate was positively associated with treatment outcome on the study level, supporting the clinical practice of reinjection to both sustain improvements and build upon them when the patient is not cured after the first injection. For example, Henalla et al. reported on 14 patients who initially failed treatment; eight (57 %) were cured or significantly improved by a second injection [21]. In addition, four patients who were significantly improved chose to undergo a second injection; three were cured and one remained significantly improved. Our findings considered with clinical evidence suggest that Macroplastique therapy may be approached as a process of treatment and re-evaluation, with the option of repeat injections if initial results are unsatisfactory and with regard to important contraindications such as a fragile urethral mucosal lining.
Though their data were too few for quantitative analysis, a small number of studies reported on patients with SUI of mixed etiology. In the randomized trial by ter Meulen and colleagues, 88 % of patients with SUI and hypermobility (n = 24) were cured or markedly improved at 12 months [10]. Findings, however, are mixed, as two studies found no effect of hypermobility on treatment efficacy [11, 19] whereas others found patients with ISD and hypermobility failed at higher rates than those with ISD alone [16, 17]. More research is required to determine the effectiveness of Macroplastique for SUI of mixed etiology.
Several randomized studies compared the relative efficacy of different UBAs primarily with collagen as the comparator. An RCT comparing Macroplastique with collagen (n = 247) observed improvement rates of 62 % and 48 % (P < 0.001) and cure rates of 37 % and 25 % (P < 0.05), respectively, after 12 months [8]. The improvement rate at 12 months for pyrolytic carbon-coated beads (80 %) was not inferior to that of collagen (69 %) in a double-blind trial (P = 0.16; n = 355) [7]. CaHA was not inferior to collagen in a single-blind trial (n = 296), with improvement rates of 63 % and 57 % at 12 months, respectively (P = 0.34) [6]. A smaller trial of CaHA and collagen (n = 46) reported 80 % improvement in the CaHA group and 62 % in the collagen group after a mean of 32 months of follow-up [36]. A single-blind RCT of dextranomer/hyaluronic acid versus collagen (n = 344) documented 12-month improvement rates of 51.2 % and 54.5 %, respectively [37]. An RCT of a copolymer of ethylene vinyl alcohol dissolved in dimethyl sulfoxide compared with collagen (n = 249) showed superior 12-month improvement rates over collagen (76 % v. 55 %; P < 0.001) [38]. A small RCT of Macroplastique and porcine dermal collagen (n = 48) reported improvement in 58 % in the porcine collagen group and 42 % in the Macroplastique group [12].
Macroplastique has a strong safety profile over 20 years, with no record of serious AEs. Importantly, there were no reports of extrusion, migration, or immune reaction in any of the papers identified in our systematic review. The most commonly identified side effects of treatment, transient dysuria and hematuria, are mild. Less frequent AEs included temporary urinary retention, temporary urge incontinence, and urinary tract infection. The RCT comparing Macroplastique with collagen provided the most comprehensive list of AEs among the reviewed publications: urinary tract infection (24 %), dysuria and urgency (9 %), frequency (8 %), and retention (7 %) [8]. These side effects are mild, transient, and easily managed without long-term sequelae for the patient compared with more invasive surgical device procedures, which require long recovery times and an increased risk of serious complications, such as severe pain, organ perforations, hemorrhage, urethral erosion, and vaginal extrusion [39].
Macroplastique is typically administered in an office-based setting with a local anesthetic, providing a simple and low-risk treatment option for women who are not candidates for general anesthesia, women who desire to get pregnant, and patients who desire a short recovery period [2]. Our analysis suggests Macroplastique is most appropriate for women who expect a long-term significant improvement in their SUI symptoms but would be satisfied if not completely cured. A recent study of women’s expectations from SUI interventions suggests most women would consider their treatment successful if they have significant improvement in symptoms (57 %) [40]. In the study of women’s expectations, 71 % of women preferred a minor procedure, such as a UBA, TVT, or transobturator tape (TOT); older women were significantly more likely to choose an office-based procedure. Other women who may prefer Macroplastique are those desiring to have more children or who would prefer a short recovery time [41].
The primary limitation of this meta-analysis is the variability in design among the included studies. Proportions and confidence intervals for some studies were observed both above and below the meta-analyzed result, which led to significant heterogeneity. Many studies had modest samples sizes, which may explain between-study variability in improvement and success rates. This may also suggest that other factors not accounted for in our analysis, such as unmeasured characteristics of the patient population, clinical definitions of cure and improvement, or clinician experience, may mitigate success rates in these studies. Another limitation is that the long-term efficacy rates had significant evidence of potential publication bias. Caution should be exercised in their interpretation, as the long-term success rate tended to be higher in larger studies than in smaller studies, whereas the long-term cure rate tended to be lower in larger studies. Finally, analyses of reinjection efficacy were based on a subset of all studies in the meta-analysis and may not have been adequately powered to detect these associations.
Four randomized trials were found, but none of the meta-analyzed statistics were able to synthesize results from all four studies. Even so, the aggregate statistics agree with and support the results found in the RCTs, with good short-term (75 %) and long-term (64 %) efficacy and safety profiles. Although the studies identified in the systematic review varied in location, design, and setting, their combined results describe a 20-year global history of safe and effective treatment with Macroplastique for adult women with SUI.
Conclusions
Results of this quantitative review support Macroplastique as an effective and durable treatment option for female SUI. Meta-analysis of all relevant peer-reviewed literature shows that Macroplastique largely sustains its therapeutic benefit in the mid-term and long-term, with some patients requiring reinjection to attain maximal effects. Macroplastique may be best suited to women who desire long-term significant improvement or cure of their SUI and who prefer a minimally invasive procedure with low morbidity and short recovery time.
References
Dooley Y, Kenton K, Cao G et al (2008) Urinary incontinence prevalence: results from the national health and nutrition examination survey. J Urol 179:656–61
American Urological Association (2009) Guideline for the Surgical Management of Female Stress Urinary Incontinence: 2009 Update. Accessed from http://www.auanet.org/content/media/stress2009-chapter1.pdf
Lovatsis D, Easton W, Wilkie D (2010) Guidelines for the evaluation and treatment of recurrent urinary incontinence following pelvic floor surgery. J Obstet Gynaecol Can 32:893–904
National Institute for Health and Clinical Excellence (2006) Urinary incontinence: the management of urinary incontinence in women. Accessed from http://www.nice.org.uk/nicemedia/live/10996/30282/30282.pdf
American Congress of Obstetricians and Gynecologists (2011) Surgery for Stress Urinary Incontinence. Accessed from http://www.acog.org/~/media/For%20Patients/faq166.pdf?dmc=1&ts=20120404T2052304770
Mayer RD, Dmochowski RR, Appell RA et al (2007) Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology 69:876–80
Lightner D, Calvosa C, Andersen R et al (2001) A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled, double-blind study of durasphere. Urology 58:12–5
Ghoniem G, Corcos J, Comiter C, Bernhard P, Westney OL, Herschorn S (2009) Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol 181:204–10
Ter Meulen PH, Berghmans LCM, van Kerrebroeck PEVA (2003) Systematic review: efficacy of silicone microimplants (Macroplastique®) therapy for stress urinary incontinence in adult women. Eur Urol 44:573–82
Ter Meulen PH, Berghmans LC, Nieman FH, van Kerrebroeck PE (2009) Effects of Macroplastique® Implantation System for stress urinary incontinence and urethral hypermobility in women. Int Urogynecol J Pelvic Floor Dysfunct 20:177–83
Maher CF, O’Reilly DPL, Carey MP, Comish A, Schluter P (2005) Pubovaginal sling versus transurethral Macroplastique for stress urinary incontinence and intrinsic sphincter deficiency: a prospective randomised controlled trial. Int J Obstet Gynaecol 112:797–801
Bano F, Barrington JW, Dyer R (2005) Comparison between porcine dermal implant (Permacol) and silicone injection (Macroplastique) for urodynamic stress incontinence. Int Urogynecol J 16:147–50
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
Snedecor G, Cochran W (1967) Statistical methods. Iowa State University Press, Ames
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Soft 36:1–48
Zullo MA, Ruggiero A, Montera R et al (2010) An ultra-miniinvasive treatment for stress urinary incontinence in complicated older patients. Maturitas 65:292–5
Plotti F, Zullo MA, Sansone M et al (2009) Post radical hysterectomy urinary incontinence: a prospective study of transurethral bulking agents injection. Gynecol Oncol 112:90–4
Zullo M, Pilotty F, Bellati F, Muzii L, Angiolo R, Panici PB (2005) Transurethral polydimethylsiloxane implantation: a valid option for the treatment of stress urinary incontinence due to intrinsic sphincter deficiency without urethral hypermobility. J Urol 173:898–902
Tamanini JT, D’Ancona CA, Netto NR Jr (2004) Treatment of intrinsic sphincter deficiency using the Macroplastique implantation system: two-year follow-up. J Endourol 18:906–11
Radley SC, Chapple CR, Mitsogiannis AC, Glass KS (2001) Transurethral implantation of Macroplastique® for the treatment of female stress urinary incontinence secondary to urethral sphincter deficiency. Eur Urol 39:383–89
Henalla SM, Hall V, Duckett JRA et al (2000) A multicentre evaluation of a new surgical technique for urethral bulking in the treatment of genuine stress incontinence. BJOG 107:1035–9
Barranger E, Fritel X, Kadoch O, Liou Y, Pigné A (2000) Results of transurethral injections of silicone microimplants for females with intrinsic sphincter deficiency. J Urol 164:1619–23
Koelbl H, Saz V, Doerfler D, Haeusler G, Sam C, Hanzal E (1998) Transurethral injection of silicone microimplants for intrinsic urethral sphincter deficiency. Obstet Gynecol 92:332–6
Mourad SM (2003) Long term efficacy of Macroplastique injection for the treatment of urinary incontinence in males and females. Eur Urol Supplements 2:175
Peeker R, Edlund C, Wennberg AL, Fall M (2002) The treatment of sphincter incontinence with periurethral silicone implants (Macroplastique). Scan J Urol Nephrol 36:194–8
Gurdal M, Tekin A, Erdogan K, Sengor F (2002) Endoscopic silicone injection for female stress urinary incontinence due to intrinsic sphincter deficiency: impact of coexisting urethral mobility on treatment outcome. Urology 60:1016–9
Soliman S, Evans C (2001) Endoscopic Macroplastique injection for the treatment of female stress incontinence: role and efficacy. African J Urol 7:45–50
Usman F, Henalla S (1998) A single transurethral Macroplastique® injection as primary treatment for stress incontinence in women. J Obstet Gynaecol 18:56–60
Sheriff MK, Foley S, Mcfarlane J, Nauth-Misir R, Shah PJ (1997) Endoscopic correction of intractable stress incontinence with silicone micro-implants. Eur Urol 32:284–8
Harriss DR, Iacovou JW, Lemberger RJ (1996) Peri-urethral silicone microimplants (Macroplastique) for the treatment of genuine stress incontinence. Br J Urol 78:722–8
Ghoniem G, Corcos J, Comiter C, Westney OL, Herschorn S (2010) Durability of urethral bulking agent injection for female stress urinary incontinence: 2-year multicenter study results. J Urol 183:1444–9
de Tayrac R, Cortesse A, Fernandez H, Fritel X (2005) Injections trans-urétrales sous anesthésie locale en ambluatoire dans l’incontinence urinaire d’effort féminine: inciations, faisabiliteé et résultats. J Gynecol Obstet Biol Reprod 34:702–710
Franceschetti GP, Bagliani F, Marchetti S, Vicini D (1998) Trattamento mini invasive della incontinenza urinaria femminile da deficit sfinterico. Chir Ital 50:17–24
Hidar S, Attyaoui F, de Leval J (2000) Injection périurétrale de microparticules de silicone dans le traitement de l’incontinence urinaire par nsuffisance spinctérienne. Prog Urol 10:219–23
Sander P, Mouritsen AL, Lose G (2004) Uretral macroplastik-injektion til behandinling af stressinkontinens hos kvinder. Ugeskr Laeger 166:3100–3102
Andersen RCM (2002) Long-term follow-up comparison of durasphere and contigen in the treatment of stress urinary incontinence. J Low Genital Tract Dis 6:239–243
Lightner D, Rovner E, Corcos J et al (2009) Randomized controlled multisite trial of injected bulking agents for women with intrinsic sphincter deficiency: mid-urethral injection of Zuidex via the Implacer versus proximal urethral injection of Contigen cystoscopically. Urology 74:771–5
Karram M, Bent A, Kennelly M, Harris T, Dmochowski R (2003) Multicenter randomized controlled study to evaluate URYX urethral bulking agent in treating female stress urinary incontinence. Int Urogynecol J 14:S62–63
Rapp DE, Kobashi KC (2008) The evolution of midurethral slings. Nat Clin Pract Urol 5:194–201
Langford CF, Elmissiry MM, Ghoniem GM (2008) Do women have realistic expectations of treatment for stress urinary incontinence? Neurourol Urodyn 27:480–484
Cespedes RD, Serkin FB (2009) Is injection therapy for stress urinary incontinence dead? No. Urology 73:11–13
Acknowledgements
We acknowledge Teresa McCabe Yurik, MS, a biostatistician with The Integra Group, for independent quality assessment, verifying accuracy of candidate inclusion, and candidate review.
Funding
Dr. Ghoniem: none. Mr. Miller: consulting support Uroplasty, Inc.
Conflicts of interest
Dr. Ghoniem: clinical study support from Uroplasty and consulting support from Boston Scientific and is a speaker for Astellas and Allergan. Mr. Miller: statistical consultant for the Integra Group, a contract research organization, and was compensated for his work on this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ghoniem, G.M., Miller, C.J. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J 24, 27–36 (2013). https://doi.org/10.1007/s00192-012-1825-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1825-9