Abstract
Purpose
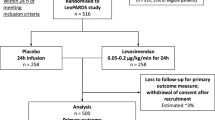
Myocardial dysfunction is common in sepsis but optimal treatment strategies are unclear. The inodilator, levosimendan was suggested as a possible therapy; however, the levosimendan to prevent acute organ dysfunction in Sepsis (LeoPARDS) trial found it to have no benefit in reducing organ dysfunction in septic shock. In this study we evaluated the effects of levosimendan in patients with and without biochemical cardiac dysfunction and examined its non-inotropic effects.
Methods
Two cardiac biomarkers, troponin I (cTnI) and N-terminal prohormone of brain natriuretic peptide (NT-proBNP), and five inflammatory mediators were measured in plasma from patients recruited to the LeoPARDS trial at baseline and over the first 6 days. Mean total Sequential Organ Failure Assessment (SOFA) score and 28-day mortality were compared between patients with normal and raised cTnI and NT-proBNP values, and between patients above and below median values.
Results
Levosimendan produced no benefit in SOFA score or 28-day mortality in patients with cardiac dysfunction. There was a statistically significant treatment by subgroup interaction (p = 0.04) in patients with NT-proBNP above or below the median value. Those with NT-proBNP values above the median receiving levosimendan had higher SOFA scores than those receiving placebo (mean daily total SOFA score 7.64 (4.41) vs 6.09 (3.88), mean difference 1.55, 95% CI 0.43–2.68). Levosimendan had no effect on the rate of decline of inflammatory biomarkers.
Conclusion
Adding levosimendan to standard care in septic shock was not associated with less severe organ dysfunction nor lower mortality in patients with biochemical evidence of cardiac dysfunction.


Similar content being viewed by others
References
Rhodes A, Evans LE, Alhazzani W et al (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. https://doi.org/10.1007/s00134-017-4683-6
Vieillard-Baron A, Caille V, Charron C et al (2008) Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 36:1701–1706. https://doi.org/10.1097/CCM.0b013e318174db05
Jardin F, Fourme T, Page B et al (1999) Persistent preload defect in severe sepsis despite fluid loading. Chest 116:1354–1359. https://doi.org/10.1378/chest.116.5.1354
Frencken JF, Donker DW, Spitoni C et al (2018) Myocardial injury in patients with sepsis and its association with long-term outcome. Circ Cardiovasc Qual Outcomes 11:1–9. https://doi.org/10.1161/CIRCOUTCOMES.117.004040
Aneman A, Vieillard-Baron A (2016) Cardiac dysfunction in sepsis. Intensive Care Med 42:2073–2076. https://doi.org/10.1007/s00134-016-4503-4
Schmittinger CA, Torgersen C, Luckner G et al (2012) Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med 38:950–958. https://doi.org/10.1007/s00134-012-2531-2
Dünser MW, Ruokonen E, Pettilä V et al (2009) Association of arterial blood pressure and vasopressor load with septic shock mortality: a post hoc analysis of a multicenter trial. Crit Care 13:R181. https://doi.org/10.1186/cc8167
Andreis DT, Singer M (2016) Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med 42:1387–1397. https://doi.org/10.1007/s00134-016-4249-z
Gordon AC, Perkins GD, Singer M et al (2016) Levosimendan for the prevention of acute organ dysfunction in sepsis. N Engl J Med 375:1638–1648. https://doi.org/10.1056/NEJMoa1609409
Ukkonen H, Saraste M, Akkila J et al (1997) Myocardial efficiency during calcium sensitization with levosimendan: a noninvasive study with positron emission tomography and echocardiography in healthy volunteers. Clin Pharmacol Ther 61:596–607. https://doi.org/10.1016/S0009-9236(97)90139-9
Wang Q, Yokoo H, Takashina M et al (2015) Anti-inflammatory profile of levosimendan in cecal ligation-induced septic mice and in lipopolysaccharide-stimulated macrophages. Crit Care Med 43:e508–e520. https://doi.org/10.1097/CCM.0000000000001269
Hasslacher J, Bijuklic K, Bertocchi C et al (2011) Levosimendan inhibits release of reactive oxygen species in polymorphonuclear leukocytes in vitro and in patients with acute heart failure and septic shock: a prospective observational study. Crit Care 15:R166. https://doi.org/10.1186/cc10307
Parissis JT, Adamopoulos S, Antoniades C et al (2004) Effects of levosimendan on circulating pro-inflammatory cytokines and soluble apoptosis mediators in patients with decompensated advanced heart failure. Am J Cardiol 93:1309–1312. https://doi.org/10.1016/j.amjcard.2004.01.073
Groesdonk H, Sander M, Heringlake M (2017) Levosimendan in sepsis. N Engl J Med 376:798–800. https://doi.org/10.1056/nejmc1616632
Orme RML, Perkins GD, McAuley DF et al (2014) An efficacy and mechanism evaluation study of Levosimendan for the Prevention of Acute oRgan Dysfunction in Sepsis (LeoPARDS): protocol for a randomized controlled trial. Trials 15:199. https://doi.org/10.1186/1745-6215-15-199
Gordon AC, Santhakumaran S, Al-Beidh F et al (2018) Levosimendan to prevent acute organ dysfunction in sepsis: the LeoPARDS RCT. Eff Mech Eval 5:1–94. https://doi.org/10.3310/eme05060
Landesberg G, Jaffe AS, Gilon D et al (2014) Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit Care Med 42:790–800. https://doi.org/10.1097/CCM.0000000000000107
Charpentier J, Luyt C-E, Fulla Y et al (2004) Brain natriuretic peptide: a marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med 32:660–665. https://doi.org/10.1097/01.ccm.0000114827.93410.d8
Post F, Weilemann LS, Messow C-M et al (2008) B-type natriuretic peptide as a marker for sepsis-induced myocardial depression in intensive care patients. Crit Care Med 36:3030–3037. https://doi.org/10.1097/CCM.0b013e31818b9153
Famous KR, Delucchi K, Ware LB et al (2017) Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 195:331–338. https://doi.org/10.1164/rccm.201603-0645OC
Ferreira FL, Bota DP, Bross A et al (2001) Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286:1754–1758
Gordon AC, Mason AJ, Perkins GD et al (2014) The interaction of vasopressin and corticosteroids in septic shock: a pilot randomized controlled trial. Crit Care Med 42:1325–1333. https://doi.org/10.1097/CCM.0000000000000212
Gordon AC, Mason AJ, Thirunavukkarasu N et al (2016) Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA 316:509–518. https://doi.org/10.1001/jama.2016.10485
Al-Mohammad A, Mant J, Laramee P et al (2010) Diagnosis and management of adults with chronic heart failure: summary of updated NICE guidance. BMJ 341:c4130. https://doi.org/10.1136/bmj.c4130
Anderson MJ, Ter Braak CJF (2003) Permutation tests for multi-factorial analysis of variance. J Stat Comput Simul 73:85–113. https://doi.org/10.1080/00949650215733
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing
Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS - A Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10:325–337. https://doi.org/10.1023/A:1008929526011
Boldt J, Menges T, Kuhn D et al (1995) Alterations in circulating vasoactive substances in the critically ill—a comparison between survivors and non-survivors. Intensive Care Med 21:218–225. https://doi.org/10.1007/BF01701475
Morelli A, Ertmer C, Westphal M et al (2013) Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock. JAMA 310:1683–1691. https://doi.org/10.1001/jama.2013.278477
Funding
The trial was funded by the Efficacy and Mechanism Evaluation Programme (11-14-08), a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership. Orion Pharma provided levosimendan and placebo free of charge. Tenax Therapeutics provided additional grant support. The NIHR Comprehensive Biomedical Research Centre (based at Imperial College Healthcare NHS Trust and Imperial College London) and the U.K. Intensive Care Foundation provided general research support. ACG is funded by an NIHR Research Professorship award (RP-2015-06-018). The funders, the sponsor (Imperial College London), Orion Pharma and Tenax Therapeutics had no role in designing the trial, gathering or analysing the data, writing the manuscript, nor making the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The views expressed in this article are those of the authors and not necessarily those of the MRC, the National Health Service (NHS), the NIHR, or the Department of Health. ACG reports receiving speaker fees from Amomed Pharma, consulting fees from Ferring Pharmaceuticals, Baxter Healthcare, Bristol-Myers Squibb and GlaxoSmithKline and grant support from HCA International, all paid to his institution; GDP receives fees for serving on an advisory board for GlaxoSmithKline; DFM receives consulting fees from Peptinnovate, Sobi, Bayer, Boehringer Ingelheim, and GlaxoSmithKline and fees to his institution from GlaxoSmithKline for participating in a clinical trial, and being named on a patent related to a new treatment for the acute respiratory distress syndrome (Canada, US, Australia, and European Union patent no., WO 2011073685, issued to Queen’s University Belfast). MS receives consulting fees and grant support from Apollo Therapeutics, Baxter, Deltex Medical, Defence Science and Technology Laboratory, GE Healthcare, Medical Technology Associates II and New Beta Innovation all paid to his institution, and heads a Data Safety Monitoring Board on behalf of Shionogi. No other potential conflict of interest relevant to this article was reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Antcliffe, D.B., Santhakumaran, S., Orme, R.M.L. et al. Levosimendan in septic shock in patients with biochemical evidence of cardiac dysfunction: a subgroup analysis of the LeoPARDS randomised trial. Intensive Care Med 45, 1392–1400 (2019). https://doi.org/10.1007/s00134-019-05731-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05731-w