Abstract
Background
Co-infection is frequently seen in critically ill patients with influenza, although the exact rate is unknown. We determined the rate of co-infection, the risk factors and the outcomes associated with co-infection in critically ill patients with influenza over a 7-year period in 148 Spanish intensive care units (ICUs).
Methods
This was a prospective, observational, multicentre study. Influenza was diagnosed using the polymerase chain reaction. Co-infection had to be confirmed using standard bacteriological tests. The primary endpoint of this analysis was the presence of community-acquired co-infection, with secondary endpoints including ICU, 28-day and hospital mortality.
Results
Of 2901 ICU patients diagnosed with influenza, 482 (16.6 %) had a co-infection. The proportion of cases of co-infection increased from 11.4 % (110/968) in 2009 to 23.4 % (80/342) in 2015 (P < 0.001). Compared with patients without co-infection, patients with co-infection were older [adjusted odds ratio (aOR) 1.1, 95 % confidence interval 1.1–1.2; P < 0.001] and were more frequently immunosuppressed due to existing HIV infection (aOR 2.6 [1.5–4.5]; P < 0.001) or preceding medication (aOR 1.4 [1.1–1.9]; P = 0.03). Co-infection was an independent risk factor for ICU mortality (aOR 1.4 [1.1–1.8]; P < 0.02), 28-day mortality (aOR 1.3 [1.1–1.7]; P = 0.04) and hospital mortality (aOR 1.9 [1.5–2.5]; P < 0.001).
Conclusions
Co-infection in critically ill patients with influenza has increased in recent years. In this Spanish cohort, age and immunosuppression were risk factors for co-infection, and co-infection was an independent risk factor for ICU, 28-day and hospital mortality.
Similar content being viewed by others
Introduction
Severe acute respiratory infection with H1N1 influenza emerged in 2009 and was associated with high mortality rates [1]. The use of early antiviral therapy was one of the cornerstones of treatment in severe respiratory infection with influenza, and was associated with better outcomes. Many patients were suspected of having a community-acquired co-infection [2]. Therefore, it was recommended to consider antibacterial treatment on admission, until an accompanying bacterial infection was excluded [3].
Previous studies suggested temporal relationships between influenza and co-infection [4]. Indeed, retrospective analysis of lung biopsies of patients who died from influenza in the pandemic of 1918 suggested bacterial super-infections of the lungs [5]. This was also found for the influenza pandemic in 1957 [6]. Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae are the most-cited bacterial causes of co-infection. However, Aspergillus spp. have also been identified as important pathogens [7]. The exact rate of co-infection and its risk factors, however, remained largely unknown. There is also a lack of understanding of the potential impact of co-infection on the outcome of patients with influenza [8].
We hypothesized that community-acquired co-infection is common and independently associated with mortality in intensive care unit (ICU) patients with influenza. Therefore, we reanalysed the data of a prospective observational study on influenza in critically ill patients in Spain from 2009 to 2015, covering four influenza seasons. In addition, we determined risk factors for co-infection.
Patients and methods
Study design
This was a prospective, observational study conducted from 2009 to 2015 in a large cohort of ICUs in Spain. There were four seasons of influenza, based on epidemic threshold rates developed by the Spanish Ministry of Health [9]: one in 2009 during the influenza H1N1 pandemic, one in the winter of 2010 to 2011, one in the winter of 2014, and one in the winter of 2015. During these four seasons (2009, 2010, 2014 and 2015), all patients admitted to the ICU with influenza-like symptoms were systematically tested to confirm respiratory infection with influenza A or bacterial pathogens. Local investigators registered data of consecutive influenza patients in a national registry created by the Spanish Society of Intensive Care Medicine. The institutional review board of Joan XXIII University Hospital approved the original study (IRBref#11,809) and waived the requirement for patients to give individual informed consent due to the observational nature of the study. The participation of 148 ICUs meant that we could monitor and prospectively follow approximately 80 % of the patients admitted to Spanish ICUs with influenza.
Inclusion and exclusion criteria
This reanalysis did not use inclusion or exclusion criteria other than those employed in the original study. However, patients under the age of 16 years and patients admitted from nursing homes or other healthcare facilities were excluded.
Standard care and collection of samples for diagnostic purposes
The Ministry of Health and competent authorities in Spain intensively monitored and audited management of influenza in the national ICUs. Standardized guidelines were used in all centres [10]. Oseltamivir therapy was considered early treatment (ET) if administered within 2 days of the onset of influenza symptoms [2], and empirical antibiotics were started after obtaining a nasopharyngeal swab, endotracheal aspirates and blood. Nasopharyngeal swabs were used for viral testing, respiratory secretions for quantitative cultures, and blood samples were cultured and used for serological tests. Bronchoalveolar lavage fluids were not obtained because of the high risk of generating aerosols. If present, pleural effusions were punctured for microbiological culture.
Definitions
Co-infection was suspected if a patient had an acute onset of signs and symptoms suggesting lower respiratory tract infection, with radiographic evidence of a pulmonary infiltrate that had no other known cause [11]. Co-infection had to be laboratory confirmed using the Centers for Disease Control and Prevention criteria. If the co-infection was diagnosed within 2 days of hospital admission, it was considered a community-acquired co-infection. The diagnosis was considered definitive if respiratory pathogens were isolated from blood or pleural fluid and if serological tests confirmed a fourfold increase of atypical pathogens, including Chlamydia spp., Coxiella burnetii and Moraxella pneumoniae. Respiratory aspergillosis was considered a ‘definite’ diagnosis only if Aspergillus spp. were identified on histopathology. The diagnosis was considered ‘probable’ if respiratory pathogens were isolated in endotracheal aspirates. Respiratory aspergillosis was considered a ‘probable’ diagnosis in the presence of halo or air-crescent signs on computed tomography of the lungs with positive determination of serum galactomannan, and ‘possible’ if Aspergillus spp. were found in endotracheal aspirates [7]. Appropriateness of antibiotic therapy was defined as administration of at least one antimicrobial agent effective against the isolated pathogen.
Study endpoints
The primary endpoint of this analysis was the presence of community-acquired co-infection. Secondary endpoints included ICU, 28-day and hospital mortality, the number of ventilator-free days and patient’s survival at day 28. Ventilator-free days were defined as days of successful and complete weaning from mechanical ventilation up to day 28. For subjects who died during this period, the ventilator-free days were counted as 0 [12].
Analysis plan
Firstly, the proportion of cases and rate of co-infection were determined. This rate was calculated per season and comparisons made among seasons. The first season acted as reference season, and calculations were carried out using logistic regression and odds ratios with confidence intervals. This was repeated for each pathogen.
Associations between co-infection and the clinical outcome measures were studied by logistic regression and corrected for potential confounders, which included gender, age, disease severity (APACHE II score), comorbidities (asthma, chronic obstructive pulmonary disease, chronic heart failure, chronic kidney disease, haematological disease, diabetes mellitus, HIV and immunodeficiency), pregnancy, obesity, oseltamivir treatment, appropriateness of initial antibiotic therapy, acute kidney injury, need for renal replacement therapy, need for invasive mechanical ventilation and presence of septic shock. Potential chronic comorbidities and states that were risk factors for the occurrence of co-infection included asthma, chronic obstructive pulmonary disease, pregnancy, obesity, diabetes mellitus, HIV and immunodeficiency and were also identified by logistic regression. Both analyses started with all potential confounders and backward selection based on P value was performed.
Statistical analysis
Discrete variables are expressed as counts with percentage and continuous variables, as means and standard deviation (SD) or as medians with the 25th to 75th interquartile range (IQR). Parametric or nonparametric tests were used for continuous variables as appropriate after the normality of the distribution had been tested. A P value <0.05 was considered significant. Differences in patients’ demographic and clinical characteristics were assessed using the Chi squared test or Fisher’s exact test for categorical variables and Student’s t test or the Mann–Whitney U test for continuous and ordinal variables, where appropriate.
Trends in the rate and proportion of cases of co-infection and causative pathogens were assessed by logistic regression, with 2009 selected as the year of reference. A stepwise backward-selection logistic regression analysis was performed to study the association with outcome. Variable selection was done based on P values (<0.10). For all models that had ICU mortality as the dependent variable, the APACHE II score was included as covariate, irrespective of the associated P value. Potential explanatory variables were checked for co-linearity prior to inclusion in the regression models using tolerance and variance inflation factor.
All statistical analysis was performed using SPSS v.20.0 for Mac (IBM Corp., Armonk, NY, USA).
Results
Patients
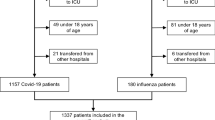
A total of 2901 ICU patients with polymerase chain reaction (PCR)-confirmed influenza were included and analysed (Table 1; Fig. 1); 1581 patients were male (59.1 %) and the mean age was 51.6 ± 15.9 years. All patients were severely ill, with a mean APACHE II score of 16.1 ± 7.6. The mean ICU and hospital length of stay were 13.5 ± 14.6 and 21.4 ± 18.8 days, respectively. ICU mortality, 28-day mortality and hospital mortality were 22.1, 19.7 and 26.2 %, respectively. S. pneumoniae was the bacterium most often identified, followed by Pseudomonas aeruginosa and methicillin-sensitive S. aureus (MSSA) (Table 2 ).
Inclusion diagram and rate of bacterial co-infection per epidemic period. Patients from four influenza epidemics were included. The total number of patients with a positive PCR for influenza was 2901. Of these, 482 had a bacterial co-infection. The lower panel gives the rate of co-infection in each period. The error bars indicate the 95 % confidence interval
Relative rate of co-infection
Overall, co-infection was diagnosed in 16.6 % of patients. An increasing trend was observed over the years of the study: 11.4 % in 2009, 17.3 % in 2010, 18.8 % in 2014, and as high as 23.4 % in 2015. The odds ratios (OR) for co-infection were 1.6 [1.2–2.2], 1.8 [1.4–2.4] and 2.4 [1.7–3.3] in 2010, 2014 and 2015 respectively (Fig. 2). A significant increase in the rates of S. pneumoniae, P. aeruginosa, MSSA and H. influenzae co-infection over the years was found (Fig. 2). The relative frequency of Aspergillus spp. did not increase over the years of the study (Fig. 2).
Odds ratios for co-infection, stratified by pathogen. Odds ratio and 95 % confidence intervals are shown per epidemic period for all co-infection (upper left) and per pathogen. The dotted line indicates an odds ratio of 1. If the error bars cross this line, the rate is not significantly different from the rate in 2009, the reference year
Risk factors for co-infection
Comorbidities in patients with and without co-infection are shown in Table 3. The likelihood of co-infection increased with age (adjusted OR 1.01 [1.01–1.02]), preceding HIV infection (adjusted OR 2.6 [1.5–4.5]) and immunosuppressive medication (adjusted OR 1.4 [1.02–1.9]). The numbers of days from onset of clinical symptoms to hospital admission, from hospital admission to start of antiviral therapy, and from onset of clinical symptoms to start of antiviral therapy did not differ between patients with and without co-infection (Supplementary Table 1) (Fig. 3).
Clinical outcomes
ICU mortality was not significantly different among influenza types (A-H1N1: 21.9 %; A-H3N2: 24.2 %; B: 18.9 %; C: 18.8 %; P = 0.7) for patients with or without co-infection. Patients with co-infection more often received early oseltamivir treatment than those without co-infection (1428/2419, 59 % vs. 314/482, 65.1 %; P = 0.01). However, early oseltamivir treatment was not associated with a significantly lower ICU mortality in patients with (171/259; 66.6 vs. 122/192; 63.5 %; P = 0.6) or without co-infection (1187/1982; 59.9 vs. 419/702; 59.7 %; P = 0.9),. Continuous renal replacement therapy, invasive mechanical ventilation and immunosuppression were independently associated with ICU mortality; the adjusted OR (aOR) values are summarized in Table 4. Co-infection was also independently associated with increased ICU mortality (aOR 1.4, 95 % CI 1.1–1.8; P < 0.02; Table 4), 28-day mortality (aOR 1.3, 95 % CI 1.1–1.7; P = 0.04) and hospital mortality (aOR 1.9 95 % CI 1.5–2.5; P < 0.001). The mean number of ventilator-free days and survival at day 28 were lower in patients with co-infection (12.9, IQR 10.6–14.2 vs. 10.3, IQR 9.6–12.1; P < 0.001). A subgroup analysis showed that only positive cultures for P. aeruginosa (aOR 2.6, 95 % CI 1.3–5.1; P = 0.004) or Aspergillus spp. (aOR 4.1, 95 % CI 1.9–9.6; P = 0.001) were independent risk factors for ICU mortality when corrected for APACHE II score.
Discussion
We have reported data from the largest prospective study to date evaluating patients with severe influenza admitted to the ICU. The most significant finding was the high rate of co-infection, complicating the clinical course in one out of six critically ill patients with influenza. Moreover, the rate of co-infection steadily increased over the study period and was independently associated with increased mortality.
Previous studies have provided conflicting results regarding the impact of co-infection on patient outcome. For example, a study performed in Europe, identifying S. pneumoniae as the most frequent pathogen isolated in co-infection, demonstrated no significant association between co-infection and ICU mortality after adjustment for confounding factors [13]. In contrast, a retrospective study analysing 683 critically ill patients in the USA with confirmed or probable 2009 influenza A, found that bacterial co-infection, especially with S. aureus, was associated with significantly higher mortality [14]. The main differences between these studies were that in the USA study only 62.1 % of the patients had confirmed co-infection and there was a higher rate of S. aureus.
All the studies published to date in critically ill patients have focused on only one influenza season, the vast majority of them on the 2009–2010 pandemic season [14–19]. Some studies also attempted to analyse the occurrence and impact of bacterial organisms complicating critical care illness during the previous 12 months [20]. In the current study we present the clinical characteristics and trend of co-infection over the past 7 years (2009–2015), providing useful information for the management of patients with severe influenza.
Studies analysing the frequency of influenza and bacterial co-infection have reported high heterogeneity. A recent systematic review and meta-analysis of 27 studies analysed the frequency of bacterial co-infection in influenza patients. The results from these studies were highly variable, ranging from 2 to 65 %, although the majority of studies ranged between 11 and 35 % [21]. Our results show a significant increase in occurrence from 11.4 % in 2009 to 23.4 % in 2015. The most frequent pathogen identified in the seven-year period was S. pneumoniae followed by P. aeruginosa and MSSA. In the last few years the rate of isolation of S. pneumoniae has been declining and the rates of P. aeruginosa and H. influenzae have increased. It is worth mentioning the reappearance of methicillin-resistant S. aureus (MRSA). When we analysed results for each pathogen individually, we found that co-infection with MSSA, P. aeruginosa and Aspergillus spp. was associated with significant mortality. These changes in epidemiology over the years may explain why, as shown in our study, co-infection has become an independent risk factor for ICU mortality.
In general, patients presenting with co-infection in our study were older, had more comorbidities (obesity, HIV and immunosuppression) and a higher severity of illness (APACHE and SOFA scores). Whilst HIV and immunosuppression were not identified as independent risk factors for co-infection in previous studies, our data show that these variables were not only associated with an increased rate of co-infection, but were also identified as risk factors for mortality in the post-pandemic period. [1, 22]. In terms of severity, patients with co-infection presented more organ failure (acute kidney injury, need for mechanical ventilation and shock). After adjusting for potentially confounding variables, the presence of co-infection was a risk factor independently associated with mortality. One important finding was the low rate of patients (4 %) with S. pneumoniae co-infection and a bacteraemic episode. Whilst the rate has commonly been reported as above 20 % in patients with community-acquired pneumonia, large multicentre studies [23] have also shown low rates (9.2 %). Bacteraemic episodes are associated with a higher fatality rate, and as a result, reports of bacteraemic episodes in patients with influenza have been less closely studied. This warrants further investigation to determine the virulence by comparing rates of bacteraemic episodes in patients with community-acquired pneumonia with and without influenza.
The delayed administration of antiviral treatment has been reported as a risk factor for ICU mortality [24]. In our study the rate of empirical administration of antiviral agents was high (70 %), and almost all patients received antiviral treatment at the time the PCR became positive (96.6 %). There were no differences in the antiviral treatment given to patients presenting with or without co-infection that could explain why co-infection patients experienced a worse outcome. Interestingly, patients with co-infection experienced a longer delay in the diagnosis of influenza and admission to ICU; however, the number of days from symptom onset to antiviral treatment was not different between those with and without co-infection. These patients may have been diagnosed initially as having community-acquired pneumonia, pending the result of a positive PCR test result for influenza. In spite of this, no difference in the number of days between admission to hospital and antiviral administration was observed between the patients with and without co-infection (5.1 days in both groups). A very surprising finding was the lack of association between appropriate antibiotic use and outcome. Appropriate antibiotic administration has been repeatedly associated with better outcomes in patients with community-acquired pneumonia [25]. Whilst co-infection was associated with worse outcome, and conversely appropriate antibiotic use did not result in better survival, we speculate that there is an unknown and complex host–pathogen interaction that can explain this finding. Another point is that among all the comorbidities, only severe immunosuppression was associated with worse outcome, supporting the major role of the immune system in the physiopathology of influenza in critically ill patients.
This study describes the clinical characteristics and outcome of the largest series of patients with confirmed RT-PCR influenza to date. The main strength of the study is its prospectively collected, consecutive design that has systematically followed up patients in 148 ICUs throughout Spain. The systematic inclusion of patients in this study and the detailed clinical characteristics recorded have allowed the Spanish healthcare system to determine and monitor patients’ characteristics, mortality rates and rate of co-infection. No other European multicentre study with prospective collection of data from critically ill patients over a period of several years has been published. We recognize that the epidemiology elsewhere may differ; however, it seems likely that in other countries around the globe have a larger population of vulnerable patients (immunosuppressed persons and the elderly) and a higher rate of co-infection than in Spain. Recent studies conducted to identify the epidemiology of pathogens in patients with either community-acquired pneumonia or healthcare-associated pneumonia showed low rates of resistant pathogens in Europe [25]. The changes in the epidemiology of co-infection demonstrated in our study therefore need to be confirmed in other countries, especially in those with higher rates of resistant pathogens.
Several limitations in the design of our study need to be acknowledged. Firstly, in 7.4 % of the patients the outcome was missing. The observational nature of the study does not allow estimation of the cause-and-effect relationship between the risk factors and outcome, as additional confounding factors may not have been identified (risk factors for healthcare-associated pneumonia, timing of antibiotic administration etc.). Of note, four episodes of Staphylococcus hominis bacteraemia might be related intravascular catheter-related infections, and diagnosis of aspergillosis was done after ICU admission in all cases but the exact date of a positive result was not captured. Co-infection diagnosis was not standardized and was based mainly on tracheal aspirate obtained immediately after intubation rather than other invasive diagnostic techniques. During the influenza periods, bronchoalveolar lavage was not systematically performed because of the high risk of generating aerosols. Bronchoscopic lavage, protected specimen brushing and transbronchial or transthoracic lung biopsies have potential risks in severely hypoxaemic intubated patients and are uncommon for standard management of patients with severe community-acquired pneumonia [26] [11]. Data on antibiotics timing and patients receiving antibiotics before bacterial sampling were not recorded as per the design of the study.
Secondly, as mentioned above, this study was restricted to Spanish ICUs, so the findings may not be applicable to non-ICU settings or to other countries. Obviously, ICU admission and criteria for endotracheal intubation were not standardized. In addition, the diagnosis of viral infection was based on nasopharyngeal swab where the determination of viral load measurement was not performed. It has been reported that nasal PCR can remain positive for weeks after clinical resolution [27]. However, significant promotion of awareness over the years by regulatory agencies such as the Centers for Disease Control and Prevention and the World Health Organization has helped physicians to treat patients promptly and adequately [28].
Conclusion
In summary, our results reveal that co-infection is diagnosed in one out of every six critically ill patients admitted to the ICU with severe influenza virus infection, with an increasing tendency over recent epidemics. Co-infection in influenza is an independent risk factor associated with higher ICU mortality because almost all patients (with or without co-infection) received antimicrobial therapy. Surprisingly, the use of appropriate antibiotic therapy was not associated with an improved outcome. The virulence of influenza and complex host–pathogen interactions in patients with co-infection deserve further attention in both epidemiological and translational research. This study is the first to show that there is a trend to more co-infection, which is independently associated with worse outcome.
References
Napolitano LM, Angus DC, Uyeki TM (2014) Critically ill patients with influenza A(H1N1)pdm09 virus infection in 2014. JAMA 311(13):1289–1290. doi:10.1001/jama.2014.2116
Rodríguez A, Díaz E, Martín-Loeches I et al (2011) Impact of early oseltamivir treatment on outcome in critically ill patients with 2009 pandemic influenza A. J Antimicrob Chemother 66:1140–1149. doi:10.1093/jac/dkq511
Martin-Loeches I, Bermejo-Martin JF, Valles J et al (2013) Macrolide-based regimens in absence of bacterial co-infection in critically ill H1N1 patients with primary viral pneumonia. Intensive Care Med 39:693–702. doi:10.1007/s00134-013-2829-8
Chertow DS (2012) Contribution of bacterial coinfection to severe influenza infection. Crit Care Med 40:1664–1665. doi:10.1097/CCM.0b013e3182451fd8
Sheng Z-M, Chertow DS, Ambroggio X et al (2011) Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc Natl Acad Sci USA 108:16416–16421. doi:10.1073/pnas.1111179108
Giles C, Shuttleworth EM (1957) Postmortem findings in 46 influenza deaths. Lancet (London, England) 273:1224–5
Martin-Loeches I, Valles J (2012) Overtreating or underdiagnosing invasive pulmonary aspergillosis (IPA) in critically ill H1N1 patients: who is right? Intensive Care Med 38:1733–1735. doi:10.1007/s00134-012-2677-y
van der Sluijs KF, van der Poll T, Lutter R et al (2010) Bench-to-bedside review: bacterial pneumonia with influenza—pathogenesis and clinical implications. Crit Care 14:219. doi:10.1186/cc8893
http://vgripe.isciii.es/gripe/inicio.do. Accessed 20 June 2016
Alfageme I, Aspa J, Bello S et al (2005) Guidelines for the diagnosis and management of community-acquired pneumonia. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Arch Bronconeumol 41:272–289
Mandell LA, Wunderink G, Anzueto A et al (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl 2):S27–S72. doi:10.1086/511159
Schoenfeld DA, Bernard GR, Network ARDS (2002) Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 30:1772–1777
Martín-Loeches I, Sanchez-Corral A, Diaz E et al (2011) Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A(H1N1) virus. Chest 139:555–562. doi:10.1378/chest.10-1396
Rice TW, Rubinson L, Uyeki TM et al (2012) Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med 40:1487–1498. doi:10.1097/CCM.0b013e3182416f23
Kakeya H, Seki M, Izumikawa K et al (2014) Efficacy of combination therapy with oseltamivir phosphate and azithromycin for influenza: a multicenter, open-label, randomized study. PLoS ONE 9:e91293. doi:10.1371/journal.pone.0091293
Martin-Loeches I, Lisboa T, Rhodes A, et al. (2010) Use of corticosteroid therapy in patients affected by severe pandemic (H1N1) V influenza A infection. Intensive Care Med. pp S319–S319
Pereira JM, Moreno RP, Matos R et al (2012) Severity assessment tools in ICU patients with 2009 influenza A (H1N1) pneumonia. Clin Microbiol Infect 18:1040–1048. doi:10.1111/j.1469-0691.2011.03736.x
Parnell GP, McLean AS, Booth DR et al (2012) A distinct influenza infection signature in the blood transcriptome of patients who presented with severe community acquired pneumonia. Crit Care 16:R157. doi:10.1186/cc11477
Martin-Loeches I, Papiol E, Rodríguez A et al (2011) Acute kidney injury in critical ill patients affected by influenza A (H1N1) virus infection. Crit Care 15:R66. doi:10.1186/cc10046
Muscedere J, Ofner M, Kumar A et al (2013) The occurrence and impact of bacterial organisms complicating critical care illness associated with 2009 influenza A(H1N1) infection. Chest 144:39–47. doi:10.1378/chest.12-1861
Klein EY, Monteforte B, Gupta A et al (2016) The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influ Other Respi Viruses 10:394–403. doi:10.1111/irv.12398
Martin-Loeches I, Díaz E, Vidaur L et al (2011) Pandemic and post-pandemic influenza A (H1N1) infection in critically ill patients. Crit Care 15:R286. doi:10.1186/cc10573
Martin-Loeches I, Lisboa T, Rodriguez A et al (2010) Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med 36:612–620
Muthuri SG, Venkatesan S, Myles PR et al (2014) Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2:395–404. doi:10.1016/S2213-2600(14)70041-4
Vallés J, Martin-Loeches I, Torres A et al (2014) Epidemiology, antibiotic therapy and clinical outcomes of healthcare-associated pneumonia in critically ill patients: a Spanish cohort study. Intensive Care Med. doi:10.1007/s00134-014-3239-2
(2014) NICE. National Institute for Health and Care Excellence. Pneumonia (including community acquired pneumonia). http://www.nice.org.uk/guidance/cg191. Accessed 20 June 2016
Estella A (2010) Bronchoalveolar lavage for pandemic influenza A (H1N1)v pneumonia in critically ill patients. Intensive Care Med 36:1976–1977. doi:10.1007/s00134-010-2009-z
Fowlkes A, Steffens A, Temte J et al (2015) Incidence of medically attended influenza during pandemic and post-pandemic seasons through the Influenza Incidence Surveillance Project, 2009-13. Lancet Respir Med 3:709–718. doi:10.1016/S2213-2600(15)00278-7
Acknowledgments
The authors are grateful to Zieta O’Hagan for language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All of the authors declare that no conflict of interest exists.
Role of funding source
The study funder (Spanish Society of Critical Care—SEMICYUC) had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author (IML) had full access to all the data in the study and final responsibility for the decision to submit for publication.
Ethics committee approval
The institutional review board of Joan XXIII University Hospital approved the original study (IRBref#11809).
Additional information
H1N1 SEMICYUC Working Group investigators are listed in Appendix section .
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix No. 1: H1N1 SEMICYUC Working Group investigators
Appendix No. 1: H1N1 SEMICYUC Working Group investigators
Andalucía : Pedro Cobo (Hospital Punta de Europa, Algeciras); Javier Martins (Hospital Santa Ana Motril, Granada); Cecilia Carbayo (Hospital Torrecardenas, Almería);Emilio Robles-Musso, Antonio Cárdenas,Javier Fierro (Hospital del Poniente, Almería); Ocaña Fernández (Hospital Huercal—Overa, Almería); Rafael Sierra (Hospital Puerta del Mar, Cádiz); Mª Jesús Huertos (Hospital Puerto Real, Cádiz); Juan Carlos Pozo, R. Guerrero (Hospital Reina Sofía, Córdoba); Enrique Márquez (Hospital Infanta Elena, Huelva); Manuel Rodríguez-Carvajal (Hospital Juan Ramón Jiménez, Huelva); Antonio Jareño, (Hospital del SAS de Jerez, Jerez de la Frontera); José Pomares, José Luis Ballesteros (Hospital Universitario San Cecilio, Granada); Yolanda Fernández, Francisco Lobato, José F. Prieto, José Albofedo-Sánchez (Hospital Costa del Sol, Marbella); Pilar Martínez (Hospital Vírgen de la Victoria, Málaga); Miguel Angel Díaz Castellanos, (Hospital Santa Ana de Motril, Granada); Guillermo Sevilla, (Clínica Sagrado Corazón, Sevilla); José Garnacho-Montero, Rafael Hinojosa, Esteban Fernández, (Hospital Virgen del Rocío, Sevilla); Ana Loza, Cristóbal León (Hospital Universitario Nuestra Señora de Valme, Sevilla); Angel Arenzana,(Hospital Virgen de la Macarena, Sevilla), Dolores Ocaña (Hospital de la Inmaculada, Sevilla) Aragón: Manuel Luis Avellanas, Arantxa Lander, S Garrido Ramírez de Arellano, MI Marquina Lacueva (Hospital San Jorge, Huesca); Pilar Luque (Hospital Lozano Blesa, Zaragoza); Ignacio González (Hospital Miquel Servet, Zaragoza); Jose Mª Montón (Hospital Obispo Polanco, Teruel); Jose Mª Díaz, Pilar López-Reina, Sergio Sáez, (Hospital Virgen de la Salud, Teruel). Asturias: Lisardo Iglesias, Carmen Pascual González (Hospital Universitario Central de Asturias—HUCA, Oviedo); Quiroga (Hospital De Cabueñes, Gijón); Águeda García-Rodríguez (Hospital Valle del Nalón, Langreo).
Baleares: Lorenzo Socias, Pedro Ibánez, Marcío Borges-Sa; A. Socias, Del Castillo A (Hospital Son LLatzer,Palma de Mallorca); Ricard Jordà Marcos (Clínica Rotger, Palma de Mallorca); José M Bonell (USP. Clínica Palmaplanas, Palma de Mallorca); Ignacio Amestarán (Hospital Son Dureta, Palma de Mallorca).
Canarias: Sergio Ruiz- Santana, Juan José Díaz, (Hospital Dr Negrín,Las Palmas de Gran Canaria); Sisón (Hospital Doctor José Molina, Lanzarote); David Hernández, Ana Trujillo, Luis Regalado, (Hospital General la Palma, La Palma); Leonardo Lorente (Hospital Universitario de Canarias, Tenerife); Mar Martín (Hospital de la Candelaria, Tenerife), Sergio Martínez, J.J.Cáceres (Hospital Insular de Gran Canaria).
Cantabria: Borja Suberviola, P. Ugarte, (Hospital Universitario Marqués de Valdecilla, Santander);
Castilla La Mancha: Fernando García-López, (Hospital General, Albacete); Angel Álvaro Alonso, Antonio Pasilla (Hospital General La Mancha Centro, Alcázar de San Juan); Mª Luisa Gómez Grande (Hospital General de Ciudad Real, Ciudad Real); Antonio Albaya, (Hospital Universitario de Guadalajara, Guadalajara); Alfonso Canabal, Luis Marina, (Hospital Virgen de la Salud, Toledo).
Castilla y León: Juan B López Messa, (Complejo Asistencial de Palencia, Palencia), Mª Jesús López Pueyo (Hospital General Yagüe, Burgos); Zulema Ferreras, (Hospital Universitario de Salamanca, Salamanca); Santiago Macias, (Hospital General de Segovia, Segovia); José Ángel Berezo, Jesús Blanco Varela, (Hospital Universitario Río Hortega, Valladolid), Andaluz Ojeda A (Hospital Universitario, Valladolid); Antonio Álvarez Terrero (Hospital Virgen de la Concha, Zamora), Fabiola Tena Ezpeleta (Hospital Santa Bárbara, Soria)
Cataluña: Rosa Mª Catalán (Hospital General de Vic, Vic); Miquel Ferrer, Antoni Torres (Hospital Clínic, Barcelona); Sandra Barbadillo (Hospital General de Catalunya—CAPIO, Barcelona); Lluís Cabré (Hospital de Barcelona, Barcelona); Assumpta Rovira (Hospital General de l’Hospitalet, L’Hospitalet); Francisco Álvarez-Lerma, Antonia Vázquez, Joan Nolla (Hospital Del Mar, Barcelona); Francisco Fernández, Joaquim Ramón Cervelló (Centro Médico Delfos, Barcelona); Rafael Mañéz, J. Ballús, Rosa Mª Granada (Hospital de Bellvitge, Barcelona); Jordi Vallés, Marta Ortíz, C. Guía (Hospital de Sabadell, Sabadell); Fernando Arméstar, Joaquim Páez (Hospital Dos De Mayo, Barcelona); Jordi Almirall,Xavier Balanzo (Hospital de Mataró, Mataró); Elena Arnau, Lluis Llopart, Mercedes Palomar (Hospital Vall d’Hebron, Barcelona); Iñaki Catalán (Hospital Sant Joan de Déu, Manresa); Josep Mª Sirvent, Cristina Ferri, Nerea López de Arbina (Hospital Josep Trueta, Girona); Mariona Badía, Montserrat Valverdú- Vidal, Fernando Barcenilla (Hospital Arnau de Vilanova, Lleida); Mònica Magret, (Hospital Sant Joan de Reus, Reus); MF Esteban, José Luna, (Hospital Verge de la Cinta, Tortosa); Juan Mª Nava, J González de Molina, (Hospital Universitario Mutua de Terrassa, Terrassa); Zoran Josic (Hospital de Igualada, Igualada); Francisco Gurri (Hospital Quirón, Barcelona); Jordi Rello, Alejandro Rodríguez, Thiago Lisboa, Diego de Mendoza, Sandra Trefler (Hospital Universitario Joan XXIII, Tarragona), Rosa María Díaz (Hospital San Camil. Sant Pere de Ribes, Barcelona)
Extremadura: Alberto Fernández-Zapata, Teresa Recio, Abilio Arrascaeta, Mª José García-Ramos, Elena Gallego (Hospital San Pedro de Alcántara, Cáceres); F. Bueno (Hospital Virgen del Puerto, Plasencia).
Galicia: Mª Lourdes Cordero, José A. Pastor, Luis Álvarez—Rocha (CHUAC, A Coruña); Dolores Vila, (Hospital Do Meixoeiro, Vigo); Ana Díaz Lamas (Hospital Arquitecto Marcide, Ferrol); Javier Blanco Pérez, M Ortiz Piquer, (Hospital Xeral—Calde, Lugo); Eleuterio Merayo, Victor Jose López-Ciudad, Juan Cortez, Eva Vilaboy (Complejo Hospitalario de Ourense, Ourense); Eva Maria Saborido, (Hospital Montecelo, Pontevedra); Raul José González, (H. Miguel Domínguez, Pontevedra); Santiago Freita, (Complejo Hospitalario de Pontevedra, Pontevedra).
La Rioja: José Luis Monzón, Félix Goñi (Hospital San Pedro, Logroño).
Madrid: Frutos Del Nogal Sáez, M Blasco Navalpotro (Hospital Severo Ochoa, Madrid); Mª Carmen García-Torrejón, (Hospital Infanta Elena, Madrid);César Pérez-Calvo, Diego López (Fundación Jiménez Díaz, Madrid); Luis Arnaiz, S. Sánchez-Alonso, Carlos Velayos, (Hospital Fuenlabrada, Madrid); Francisco del Río, Miguel Ángel González (Hospital Clínico San Carlos, Madrid); María Cruz Martín, José Mª Molina (Hospital Nuestra Señora de América, Madrid); Juan Carlos Montejo (Hospital Universitario 12 de Octubre, Madrid); Patricia Albert, Ana de Pablo (Hospital del Sureste, Arganda del rey); José Eugenio Guerrero, Jaime Benitez Peyrat (Hospital Gregorio Marañón, Madrid); José A Juliá, Enrique Cerdá, Manuel Alvarez, Carlos Pey, (Hospital Infanta Cristina, Madrid); Montse Rodríguez, Eduardo Palencia (Hospital Infanta Leonor, Madrid); Rafael Caballero, (Hospital de San Rafael, Madrid); Rafael Guerrero (Hospital Reina Sofía, Madrid); Concepción Vaquero, Francisco Mariscal, S. García, (Hospital Infanta Sofía, Madrid); Almudena Simón (Hospital Nuestra Señora del Prado, Madrid); Nieves Carrasco, (Hospital Universitario La Princesa, Madrid); Isidro Prieto, A Liétor, R. Ramos (Hospital Ramón y Cajal, Madrid);Beatríz Galván, Juan C. Figueira, M. Cruz Soriano (Hospital La Paz, Madrid); P Galdós; Bárbara Balandin Moreno (Hospital Puerta de Hierro, Madrid); Fernández del Cabo (Hospital Monte Príncipe, Madrid); Cecilia Hermosa, Federico Gordo (Hospital de Henares, Madrid); Alejandro Algora (Hospital Universitario Fundación Alcorcón, Madrid); Amparo Paredes (Hospital Sur de Alcorcón, Madrid); JA Cambronero (Hospital Universitario Príncipe de Asturias, Madrid); Sonia Gómez-Rosado, (Hospital de Móstoles, Madrid).
Murcia:
Sofía Martínez (Hospital Santa María del Rosell, Murcia); F. Felices Abad, (Hospital Universitario Reina Sofía, Murcia);Mariano Martinez, (Hospital Universitario Virgen de la Arrixaca, Murcia); Sergio Manuel Butí, Gil Rueda, Francisco García(Hospital Morales Messeguer, Murcia.
Navarra: Laura Macaya, Enrique Maraví-Poma, I Jimenez Urra, L Macaya Redin, A Tellería (Hospital Virgen del Camino, Pamplona); Josu Insansti, (Hospital de Navarra, Pamplona).
País Vasco: Nagore González, Pilar Marco, Loreto Vidaur (Hospital de Donostia, San Sebastián); B. Santamaría, (Hospital de Basurto, Bilbao); Juan Carlos Vergara, Jose Ramon Iruretagoyena Amiano, (Hospital de Cruces, Bilbao); Alberto Manzano, (Hospital Santiago Apóstol, Vitoria); Carlos Castillo Arenal (Hospital Txagorritxu, Vitoria).
Valencia: José Blanquer (Hospital Clinic Universitari, Valencia); Roberto Reig Valero, A. Belenger, Susana Altaba (Hospital General de Castellón, Castellón); Bernabé Álvarez -Sánchez, (Hospital General de Alicante, Alicante); Santiago Alberto Picos, (Hospital Torrevieja Salud, Alicante); Ángel Sánchez-Miralles, (Hospital San Juan, Alicante); Juan Bonastre, M. Palamo, Javier Cebrian, José Cuñat (Hospital La Fe, Valencia); Belén Romero (Hospital de Manises, Valencia); Rafael Zaragoza, (Hospital Dr Peset, Valencia); Virgilio Paricio, (Hospital de Requena, Valencia); Asunción Marques, S. Sánchez-Morcillo, S. Tormo (Hospital de la Ribera, Valencia). J. Latour (H.G Universitario de Elche, Valencia), M Ángel García (Hospital de Sagunto, Castellón).
Rights and permissions
About this article
Cite this article
Martin-Loeches, I., J Schultz, M., Vincent, JL. et al. Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med 43, 48–58 (2017). https://doi.org/10.1007/s00134-016-4578-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4578-y