Abstract
In patients with acute respiratory distress syndrome (ARDS), in supine position, there is a decrease of inflation along the sternum vertebral axis, up to lung collapse. In 1991 we published a report showing that, in ARDS patients, shifting from supine to prone position led immediately to the inversion of the inflation gradient and to a redistribution of densities from dorsal to ventral lung regions. This led to a “sponge model” as a wet sponge, similar to a heavy edematous lung, squeezes out the gas in the most dependent regions, due to the weight-related increase of the compressive forces. The sponge model accounts for density distribution in prone position, for which the unloaded dorsal regions are recruited, while the loaded ventral region, collapses. In addition, the sponge model accounts for the mechanism through which the positive end-expiratory pressure acts as counterforce to oppose the collapsing, compressing forces.
The final result of proning was that the inversion of gravitational forces, together with other factors such as lung-chest wall shape-matching and the heart weight led to a more homogeneous distribution of inflation throughout the lung parenchyma. This is associated with oxygenation improvement as the dorsal recruitment, for anatomical reasons, prevails on the ventral de-recruitment. The more homogeneous distribution of inflation (i.e. of stress and strain) decreases/prevents the ventilator-induced lung injury, as consistently shown in animal experiments. Finally, and a series of clinical trials led to the conclusion that in patients with severe ARDS, the prone position provides a significant survival advantage.
Similar content being viewed by others
Introduction
In 1991, we published a study in Anesthesiology in which we used quantitative CT scan analysis to compare the effects of prone and supine positioning in patients with acute respiratory distress syndrome (ARDS) [1]. The main finding was that, unexpectedly, the lung densities in prone position redistributed from dorsal to ventral regions, but the average lung density remained unchanged. Twenty-two years later, we have been offered the privilege of discussing the overall impact of this article on our scientific community and its fallout in clinical practice. We must first position that study in the time frame in which it was conceived, designed, conducted and analyzed. The findings and analysis of that article led to the development of the ARDS “sponge lung” model [2], which best explains the density redistribution and, in the following years, provided the pathophysiological background to explain the effects of prone position on the stress and strain distribution throughout the lung parenchyma and its possible beneficial impact on outcome. In addition, the “sponge model,” originating from this article, accounted for lung recruitability, gas exchange and finally the primary mechanisms of positive end-expiratory pressure (PEEP).
The time frame
Rediscovering the prone position
The prone position, to our knowledge, was first described in the intensive care literature in two papers: a theoretical one by Bryan et al. [3] stressed its potential beneficial impact on lung mechanics, and a second one by Piehl et al. [4] reported dramatic effects on oxygenation improvement. Unfortunately, as often happens when times are not ripe, these potentially seminal papers were substantially overlooked, with no impact at all on the community, which was not ready to accept their conclusions.
Years later, independently, in the same month both Maunder [5] and our group [6] reported studies focused on the findings from chest CT scans in patients with ARDS. Maunder et al. called attention to the preservation of normal regions of the lung, while we were more surprised by its non-homogeneity. With CT scans we could define the “amount of disease,” represented by non-aerated tissue, the “working lung,” represented by well-aerated tissue, and an intermediate zone that we called poorly inflated tissue. When we compared the anatomical characteristics of the ARDS patients with the physiological variables, the main finding was that the amount of well-aerated tissue was closely related to the compliance of the respiratory system, indicating that the lung was not stiff but rather small [7]. This view developed into the baby lung model. Accordingly, in an adult with ARDS, the “working” ventilatable lung has the dimensions of the lung of a 3–6-year-old child.
As the densities representing atelectasis/consolidated tissue were mainly in the dependent lung regions, the baby lung was obviously located, in supine position, in the ventral regions. Therefore, our model of ARDS, in the late 1980s, consisted of an anatomically well-defined baby lung in the ventral regions, which accounted for the reduced lung compliance and provided the gas exchange associated with a mass of collapsed diseased/edematous tissue in the dorsal lung regions, unventilated and the cause of venous admixture [8, 9]. It thus became quite natural for us, when the patient came back from the CT scan facilities to the intensive care unit, to put him in the prone position, aiming to redistribute the blood flow from the posterior unventilated lung to the now dependent baby lung. This maneuver often achieved spectacular increases in oxygenation.
In 1988, the curiosity of a young doctor wanting to prove the validity of the flow redistribution hypothesis led him to take a CT scan with the patient in prone position.
To his and our absolute surprise, when we were called to see the CT scan, we saw that the baby lung had disappeared from the ventral zone and a new baby lung had appeared in the dorsal regions with no changes in the pressures at which the CT scan had been taken. This chance finding, which we reported in Chest [10], induced us to organize a study to confirm the density redistribution prospectively and introduce regional analysis of the lung parenchyma to understand the mechanism. We must bear in mind that at that time phenomena such as atelectrauma, biotrauma and stress/strain distribution were largely unknown.
The sponge lung model
The most immediate possible explanation for the density redistribution on shifting the patient from the supine to prone position is the shift of the edema, with gravity, throughout the lung parenchyma. This, however, contrasts with the fact that the dense lung edema cannot flow freely through tissues, especially considering that lung density redistributes in the few minutes necessary to take a CT scan in prone position. If the density redistribution cannot be attributed to an excess of fluid in the dependent regions, the only explanation is that it is due to a decrease in gas content. In fact, we must remember that densities computed from CT scan analysis are the ratio of the tissue mass to the tissue volume (nearly identical to tissue mass as their ratio is nearly 1) plus gas volume.
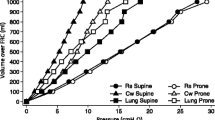
To prove that this was the mechanism, we had to investigate the regional tissue mass distribution, noting that “tissue mass” includes the parenchyma, blood, edema and cell debris present in the lung. Therefore, we divided the lung parenchyma into ten levels from ventral to dorsal, computing the CT characteristics of each level. In each level, the amount of tissue mass in ARDS patients was almost double that in normal subjects, indicating that excess tissue mass, likely the edema, is quite evenly distributed, at least in patients with severe ARDS (Fig. 1). Therefore, our explanation for the density redistribution was that the increased weight of the non-dependent lung regions, which we quantified with superimposed pressure, progressively compressed the dependent lung regions along the gravitational axis. This concept was later summarized as “the sponge model” in an editorial in JAMA [2]. Accordingly, the size of the sponge holes decreases along the vertical axis when the sponge is wet compared to dry. Therefore, although the ARDS lung appears non-homogeneous at CT scan because of its uneven state of inflation, its excess tissue mass appears homogeneously distributed [11]. Moreover, the inflammatory cell activity appears present throughout the whole lung, independent of the inflation status, as shown by positron emission tomography [12]. This mechanism is at work in normal subjects too, causing a well-known reduction in alveolar size in dependent lung regions. In ARDS, where the lung is 2–3 times heavier than normal, in supine position the potentially open alveoli in the dorsal dependent region collapse. In prone position they can open up as the superimposed pressure of the dorsal regions is released while the ventral regions are compressed. This mechanism is also the basis for the action of PEEP, which works as a counterforce to gravity [13].
The gas:tissue ratio as a function of lung height in the supine (rhombus) and prone (circles) positions. The white symbols refer to normal lungs (14 patients) and the black symbols to lungs of patients with adult respiratory distress syndrome (ARDS) (20 patients). The height = 0 refers to the ventral surface in the supine position and to the dorsal surface in the prone position. The height scale direction is from nondependent (0) to dependent (100). Reproduced from Ref. [11] with permission
Therefore, the findings from the study we are revisiting led to the introduction of concepts such as edema distribution, superimposed pressure and PEEP action, and later on—as we will discuss—to stress and strain distribution, gas exchange behavior and possible clinical outcome benefits.
Prone position and gas exchange
The first report documenting an improvement in oxygenation was consistently confirmed by all the studies using prone position [14], and in a substantial portion of patients the oxygenation improvement persisted even after returning to the supine position [15, 16]. This appears purely due to mechanical forces, as has been observed not only in ARDS, but also hydrostatic edema [17, 18], after surgery and trauma. In the article in Anesthesiology, we showed that the decrease in density in the dorsal regions was associated with increasing density in the ventral regions, leaving the average density of the lung unchanged.
The improvement in oxygenation associated with recruitment, however, is only possible if the de-recruitment of the ventral regions does not offset the recruitment of the dorsal ones. Let’s consider, as an example, an elliptical-shaped lung, in supine position, in which the gravitational forces would cause a complete collapse of the dorsal-dependent half. When turned prone, the dorsal half, previously collapsed, will open, while the ventral half, previously open, will collapse. However, as the amount of open and closed mass will be the same in both positions, if the perfusion does not change, the venous admixture will remain the same as well as the oxygenation. In contrast, in a triangular-shaped lung, the dorsal regions include more tissue mass than the ventral ones. Therefore, in prone position, if the lung collapse occurs at the same middle level, the tissue that regains aeration is more than the collapsed tissue (see Fig. 2). Consequently, with unchanged perfusion, the venous admixture will be lower in prone position and the oxygenation will be better. This fluid-like “sponge model” based on the superimposed pressure has been challenged by other authors who explained the density differences by the necessity of matching two structures, the lung (cone) and the chest wall (cylinder), with different shapes. Accordingly, the tip of the cone in supine position will expand to match the cylinder, while in prone position it would accommodate the changes of the chest wall shape due to gravity. Other factors, however, may affect the density redistribution, such as the abdominal pressure [19, 20] and the heart weight [21, 22]. All these factors are likely involved to different extents in inducing density redistribution and explain why the decrease in density in prone position along the vertical axis is smoother than its decrease in supine position. In fact, the rate of density decrease in a fluid-like lung would be identical in both positions. It must be noted, however, that among all the factors that may contribute to the densities redistribution, the one that changes more in ARDS is the lung mass, i.e., the superimposed pressure. In summary, whatever the true explanation is, one fact holds true: in prone position, the density redistribution in the lung behaves more or less as a wet sponge. As a consequence, a greater open lung mass is available in prone position [11].
Changes in the shape of the lung on shifting from the supine to the prone position, quantified by dividing the area in two (at 50 % of the ventral/dorsal distance), the upper (U) and lower (L) compartments, and calculating the U/(U + L) ratios in both positions. The difference in U/(U + L) ratios between the prone and supine positions (ΔU/(U + L) ratio) gives a quantitative indication of the change in lung shape. Examples show a perfectly symmetrical lung (ellipse, upper panel) with ΔU/(U + L) = 0 and a triangular lung with ΔU/(U + L) = 50 %
That ventral de-recruitment does not offset dorsal recruitment has been shown in animal models [23] in which ventilation was greater in the dorsal regions in prone position, with the perfusion evenly distributed, as also documented with the microsphere technique by Walther et al. [24]. Therefore, the primary mechanism of oxygenation improvement appears to depend on dorsal recruitment being greater than ventral de-recruitment, with unmodified lung perfusion.
In general, vasoconstriction does not appear to change in the prone patient; consequently, nitric oxide inhalation—in all the studies in which it was tested—had an additive effect on oxygenation [25–28]. Less attention has been paid to the CO2 clearance than oxygenation, although its improvement has been associated with greater recruitment [29] and better outcome [30]. It is worth emphasizing that CO2 clearance, as assessed by physiological dead space, is related to structural lung alterations [31] and final outcome [32] far more than oxygenation impairment.
Prone position and lung mechanics
Total respiratory system
The immediate effects of prone positioning are on the chest wall as, for anatomical reasons, its dorsal part is stiffer than the ventral part. The use of supports to ensure a free abdomen in the prone position, although theoretically interesting, was useless in adults, where gas exchange and chest wall mechanics were similar with or without supports [33]. In children, however, supports gave different results [34]. Given the decrease of chest wall compliance in prone position [35, 36], if lung compliance stays the same, the total respiratory system compliance decreases. However, if the lung compliance increases because of lung recruitment, the total respiratory system compliance may stay constant or even increase depending on the extent of recruitment. Stiffening the thoracic part of the chest wall may increase the distribution of the tidal volume toward the diaphragmatic regions of the lung, as opposed to what happens in the supine position.
Prone position and stress/strain distribution
The main reason why the prone position should affect the outcome, beyond the rescue of oxygenation impairment, is that it should make mechanical ventilation less dangerous, reducing the likelihood of ventilator-induced lung injury. In the article in Anesthesiology, we showed that inflation is more homogeneous in the prone than in the supine position. Figure 1 shows that normal subjects had a ventral gas:a tissue ratio in supine position of 6, and this decreased almost linearly down to 2.5 in the dorsal regions. If we accept that the ventral and dorsal parenchymas of the lung are similar, this means that the forces applied to distend the ventral regions are twice those applied to the dorsal ones, i.e., their strain is double and consequently their stress too. In the prone position the gas:tissue ratio does not exactly mirror the supine distribution, suggesting that forces other than gravitational are operating, too. In spite of this, the gas:tissue ratio in the dorsal, now non-dependent regions was 4.5 compared to 3.5 in the ventral regions, now dependent. Consequently, the regional difference in baseline stress and strain is much less in the prone position compared to the supine position.
In ARDS patients, the distribution of stress and strain is similar to that of normal subjects even though in general the gas:tissue ratio is lower because of the increased lung mass. More homogeneous inflation distribution in prone patients has been reported by various investigators [37, 38] and using techniques different from CT scans [23]. If the baseline parenchyma condition is more homogeneous in prone position and, in addition, prone position is associated with greater recruitment (more open alveoli), it follows that the ventilation is less harmful as stress and strain are more evenly distributed throughout the lung parenchyma. Animal experiments clearly showed that mechanical ventilation in the prone position causes less lung damage than in the supine position [39–42], or at least delays its appearance.
Prone position: clinical studies
A number of large studies (Table 1) and meta-analyses [43–45] have been dedicated to this aspect of respiratory therapy. In the first randomized study, which compared the prone to supine position, published in 2001 but designed at least 4 years earlier (Table 2), better outcome was only expected on the basis of the assumption that better oxygenation would be associated with better outcome [46]. Nowadays, this approach clearly seems naïf, but at that time, concepts such as biotrauma, the lack of correspondence between oxygenation and outcome, lung protective strategies and the whole philosophy of those protective strategies were in their “infancy” or not even born. This first study, although confirming the possible effects of prone position on oxygenation, with marginal side effects, did not find any outcome benefits. However, with all the limitations of post hoc analysis, it was clear that in the most severe ARDS cases (a quartile of lowest PaO2/FiO2 corresponding to the current definition of severe ARDS), the mortality rate was almost half in the prone compared to the supine position. In subsequent years, accumulating evidence of the benefits of gentle treatment of the baby lung, experimental evidence indicating less ventilator-induced lung injury in different animals treated in prone compared to supine position and clinical suggestions of benefits—while not reaching statistical significance [47]—led us to design a new prone-supine study [48]. The rationale shifted from oxygenation to lung protection by a more homogeneous distribution of stress and strain, which should make the mechanical ventilation less harmful. Mechanical ventilation parameters were controlled and the time allowed in prone position was no longer limited to a few hours per day but as long as 20 h. The results, however, did not show a significant benefit on outcome, although post hoc analysis nevertheless indicated advantages of the prone position in severe ARDS.
A large French study including not only ARDS but also hypoxemic respiratory failure once again proved beneficial effects on oxygenation but not on mortality [49]. However, a meta-analysis [43] and an individual patient study [44] collecting the main studies published at the time clearly suggested some survival advantage in severe ARDS, as defined by a PaO2/FiO2 ratio lower than 100 mmHg.
Finally, at the time of this writing, Guerin et al. [50] have provided the first randomized trial showing an impressive survival benefit—about 50 %—in patients included with PaO2/FiO2 lower than 150 mmHg, FiO2 at least 0.6, PEEP at least 5 cm H2O and tidal volume close to 6 ml per kilogram of predicted body weight.
Conclusion
We believe that few therapeutic adventures in critical care such as the prone position in ARDS have had such a meaningful and logical course. Everything started with a chance observation. CT scans provided a strong anatomical and pathological background for understanding the effects of the prone position on making mechanical ventilation less harmful. A series of increasingly refined randomized trials and meta-analyses strongly indicated that we can expect a 10 % survival advantages in the patient population with severe ARDS treated in prone position. Finally, a clear-cut randomized trial—by Guerin et al.—completed this 30-year course showing unquestionable survival advantages. We can now recommend the prone position as an effective routine tool in severe ARDS patients.
References
Gattinoni L, Pelosi P, Vitale G, Pesenti A, D’A L, Mascheroni D (1991) Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure. Anesthesiology 74:15–23
Bone RC (1993) The ARDS lung: New insights from computed-tomography. JAMA 269:2134–2135
Bryan AC (1974) Comments of devil’s advocate (editorial). Am Rev Respir Dis 110(Suppl):143–144
Piehl MA, Brown RS (1976) Use of extreme position changes in acute respiratory failure. Crit Care Med 4:13–14
Maunder RJ, Shuman WP, McHugh JW, Marglin SI, Butler J (1986) Preservation of normal lung regions in the adult respiratory distress syndrome: analysis by computed tomography. JAMA 255:2463–2465
Gattinoni L, Presenti A, Torresin A, Baglioni S, Rivolta M, Rossi F et al (1986) Adult respiratory distress syndrome profiles by computed tomography. J Thorac Imaging 1:25–30
Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M (1987) Pressure-volume curve of total respiratory system in acute respiratory failure: computed tomographic scan study. Am Rev Respir Dis 136:730–736
Gattinoni L, Pesenti A (1987) ARDS: the non-homogeneous lung; facts and hypothesis. Intensive Crit Care Dig 6:1–4
Gattinoni L, Pesenti A (2005) The concept of “baby lung”. Intensive Care Med 31:776–784
Langer M, Mascheroni D, Marcolin R, Gattinoni L (1988) The prone position in ARDS patients: a clinical study. Chest 94:103–107
Gattinoni L, Taccone P, Mascheroni D, Valenza F, Pelosi P (2013) Prone positioning in acute respiratory failure. Principles And Practice of Mechanical Ventilation, 3rd edn. McGraw Hill pp. 1169-1182
Bellani G, Messa C, Guerra L, Spagnolli E, Foti G, Patroniti N et al (2009) Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: a [18F]-fluoro-2-deoxy-d-glucose PET/CT study. Crit Care Med 37:2216–2222
Gattinoni L, Dandrea L, Pelosi P, Vitale G, Pesenti A, Fumagalli R (1993) Regional effects and mechanism of positive end-expiratory pressure in early adult respiratory-distress syndrome. JAMA 269:2122–2127
Alsaghir AH, Martin CM (2008) Effect of prone positioning in patients with acute respiratory distress syndrome: a meta-analysis. Crit Care Med 36:603–609
Chatte G, Sab JM, Dubois JM, Sirodot M, Gaussorgues P, Robert D (1997) Prone position in mechanically ventilated patients with severe acute respiratory failure. Am J Respir Crit Care Med 155:473–478
Lee DL, Chiang HT, Lin SL, Ger LP, Kun MH, Huang YCT (2002) Prone-position ventilation induces sustained improvement in oxygenation in patients with acute respiratory distress syndrome who have a large shunt. Crit Care Med 30:1446–1452
Nakos G, Tsangaris I, Kostanti E, Nathanail C, Lachana A, Koulouras V et al (2000) Effect of the prone position on patients with hydrostatic pulmonary edema compared with patients with acute respiratory distress syndrome and pulmonary fibrosis. Am J Respir Crit Care Med 161:360–368
Venet C, Guyomarc’h S, Migeot C, Bertrand M, Gery P, Page D et al (2001) The oxygenation variations related to prone positioning du ventilation: a clinical ring mechanical comparison between ARDS and non-ARDS hypoxemic patients. Intensive Care Med 27:1352–1359
Rouby JJ, Puybasset L, Nieszkowska A, Lu Q (2003) Acute respiratory distress syndrome: lessons from computed tomography of the whole lung. Crit Care Med 31:S285–S295
Mure M, Glenny RW, Domino KB, Hlastala MP (1998) Pulmonary gas exchange improves in the prone position with abdominal distension. Am J Respir Crit Care Med 157:1785–1790
Malbouisson LM, Busch CJ, Puybasset L, Lu Q, Cluzel P, Rouby JJ et al (2000) Role of the heart in the loss of aeration characterizing lower lobes in acute respiratory distress syndrome. Am J Respir Crit Care Med 161:2005–2012
Albert RK, Hubmayr RD (2000) The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med 161:1660–1665
Richter T, Bellani G, Harris RS, Melo MFV, Winkler T, Venegas LG et al (2005) Effect of prone position on regional shunt, aeration, and perfusion in experimental acute lung injury. Am J Respir Crit Care Med 172:480–487
Walther SM, Domino KB, Glenny RW, Hlastala MP (1999) Positive end-expiratory pressure redistributes perfusion to dependent lung regions in supine but not in prone lambs. Crit Care Med 27:37–45
Papazian L, Bregeon F, Gaillat F, Thirion X, Gainnier M, Gregoire R et al (1998) Respective and combined effects of prone position and inhaled nitric oxide in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 157:580–585
Martinez M, Diaz E, Joseph D, Villagra A, Mas A, Fernandez R et al (1999) Improvement in oxygenation by prone position and nitric oxide in patients with acute respiratory distress syndrome. Intensive Care Med 25:29–36
Borelli M, Lampati L, Vascotto E, Fumagalli R, Pesenti A (2000) Hemodynamic and gas exchange response to inhaled nitric oxide and prone positioning in acute respiratory distress syndrome patients. Crit Care Med 28:2707–2712
Rialp G, Betbese AJ, Perez-Marquez M, Mancebo J (2001) Short-term effects of inhaled nitric oxide and prone position in pulmonary and extrapulmonary acute respiratory distress syndrome. Am J Respir Crit Care Med 164:243–249
Vieillard-Baron A, Rabiller A, Chergui K, Peyrouset O, Page B, Beauchet A et al (2005) Prone position improves mechanics and alveolar ventilation in acute respiratory distress syndrome. Intensive Care Med 31:220–226
Protti A, Chiumello D, Cressoni M, Carlesso E, Mietto C, Berto V et al (2009) Relationship between gas exchange response to prone position and lung recruitability during acute respiratory failure. Intensive Care Med 35:1011–1017
Gattinoni L, Bombino M, Pelosi P, Lissoni A, Pesenti A, Fumagalli R et al (1994) Lung structure and function in different stages of severe adult respiratory distress syndrome. JAMA 271:1772–1779
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD et al (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346:1281–1286
Chiumello D, Cressoni M, Racagni M, Landi L, Li BG, Polli F et al (2006) Effects of thoraco-pelvic supports during prone position in patients with acute lung injury/acute respiratory distress syndrome: a physiological study. Crit Care 10:R87
Ungern-Sternberg BS, Hammer J, Frei FJ, Jordi Ritz EM, Schibler A, Erb TO (2007) Prone equals prone? impact of positioning techniques on respiratory function in anesthetized and paralyzed healthy children. Intensive Care Med 33:1771–1777
Pelosi P, Tubiolo D, Mascheroni D, Vicardi P, Crotti S, Valenza F et al (1998) Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med 157:387–393
Guerin C, Badet M, Rosselli S, Heyer L, Sab JM, Langevin B et al (1999) Effects of prone position on alveolar recruitment and oxygenation in acute lung injury. Intensive Care Med 25:1222–1230
Galiatsou E, Kostanti E, Svarna E, Kitsakos A, Koulouras V, Efremidis SC et al (2006) Prone position augments recruitment and prevents alveolar overinflation in acute lung injury. Am J Respir Crit Care Med 174:187–197
Cornejo RA, Diaz JC, Tobar EA, Bruhn AR, Ramos CA, Gonzalez RA et al. (2013) Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med
Du HL, Yamada Y, Orii R, Suzuki S, Sawamura S, Suwa K et al (1997) Beneficial effects of the prone position on the incidence of barotrauma in oleic acid-induced lung injury under continuous positive pressure ventilation. Acta Anaesthesiol Scand 41:701–707
Broccard A, Shapiro RS, Schmitz LL, Adams AB, Nahum A, Marini JJ (2000) Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Crit Care Med 28:295–303
Nishimura M, Honda O, Tomiyama N, Johkoh T, Kagawa K, Nishida T (2000) Body position does not influence the location of ventilator-induced lung injury. Intensive Care Med 26:1664–1669
Valenza F, Guglielmi M, Maffioletti M, Tedesco C, Maccagni P, Fossali T et al (2005) Prone position delays the progression of ventilator-induced lung injury in rats: does lung strain distribution play a role? Crit Care Med 33:361–367
Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NKJ, Latini R et al (2010) Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 36:585–599
Gattinoni L, Carlesso E, Taccone P, Polli F, Guerin C, Mancebo J (2010) Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol 76:448–454
Abroug F, Ouanes-Besbes L, Dachraoui F, Ouanes I, Brochard L (2011) An updated study-level meta-analysis of randomised controlled trials on proning in ARDS and acute lung injury. Critical Care 15
Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V et al (2001) Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 345:568–573
Mancebo J, Fernandez R, Blanch L, Rialp G, Gordo F, Ferrer M et al (2006) A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 173:1233–1239
Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C et al (2009) Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 302:1977–1984
Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P et al (2004) Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA 292:2379–2387
Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T et al (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368:2159–2168
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gattinoni, L., Pesenti, A. & Carlesso, E. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure: impact and clinical fallout through the following 20 years. Intensive Care Med 39, 1909–1915 (2013). https://doi.org/10.1007/s00134-013-3066-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-3066-x